The most common aliphatic polyamines belong to the homologous series of diethylene triamine (DETA), triethylenetetramine (TETA), and tetraethylenepentamine (TEPA), which contain both primary and secondary amine groups.
Aliphatic polyamines (Figure 2.45) cure epoxy resins at a fast rate and produce a densely cross-linked network with very good chemi-
|
|
cal resistance. However, they suffer from such limitations as short pot life, poor flexibility, poor impact resistance, and more importantly, high volatility, toxicity and potential for skin sensitization. They also have the tendency to produce blushing (amine bloom) when used in high humidity and low temperature conditions.
Cycloaliphatic amines (Figure 2.46) are less volatile than aliphatic polyamines but are still considered skin sensitizing agents. Unless modified by acid accelerators such as salicylic acid, they require higher temperature for full curing. Other important cyclic curatives are N-aminoethylpiperazine and m-xylylenediamine; the latter has aliphatic amines attached to the aromatic ring and therefore gives the typical performance advantages of aromatic and cycloaliphatic amines.
|
Figure 2.46: Cycloaliphatic amines: (a) isophorone diamine, (b) 1,2-diamino — cyclohexane, (c) 4,4’-diaminodicyclohexylmethane, (d) N-aminoethylpipera — zine, (e) m-xylylenediamine |
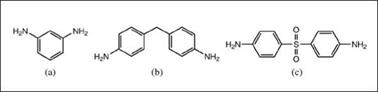
Aromatic amines (Figure 2.47) are less reactive than aliphatic amines and require higher curing temperatures. They yield rigid networks with superior chemical and heat resistance, but their dark color limits their applications.
|
Figure 2.47: Aromatic amines: (a) m-phenylenediamine, (b) 4,4’-diaminodi- phenylmethane, (c) 4,4’-diaminodiphenylsulfone |
Polyoxyalkylene amines (polyglycol amines) are another important group of polyamine hardeners. Chemically they are amine terminated polyethers derived from polyethylene glycols or polypropylene glycols. Among their unique features are flexibility, longer pot life and lighter color.
In order to address the issues of volatility and toxicity associated with low MW aliphatic amines, different chemical modifications are adopted to derive amine cross-linkers with higher MW and hence lower volatility. Some commercially important derivatives are polyamine adducts, amine terminated polyamides, amidoamines, Mannich bases, and phenalkamines.
Polyamine adducts (Figure 2.48) are the products (adducts) prepared by using excess equivalents of a polyamine (such as DETA and TETA) to a standard epoxy resin. Such amine-functional products have higher MW and lower volatility, making them good candidates for amine type curing agents. Their final film properties are similar to those obtained by polyamines, but they have a reduced tendency for blushing.
 10 октября, 2015
10 октября, 2015  Pokraskin
Pokraskin 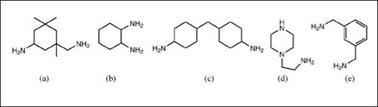
 Опубликовано в рубрике
Опубликовано в рубрике 