3 ‘-Chlorofluorans react with a wide variety of primary amines such as alkylamines, cycloalkylamines, aralkylamines, and arylamines, as well as cyclic secondary amines such as piperidine, morpholine, etc., to prepare 3’-aminofluorans.
The typical example is, however, the reaction of 3′-chlorofluoran (84) with cyclohexylamine to give 3′-cyclohexylaminofluorans (85) (Eq. 8).
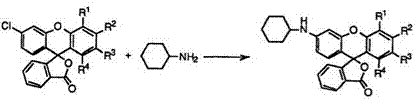 |
The reaction is successfully carried out at 180-220°C in the presence of zinc chloride and zinc oxide. When 3′,6′-dichlorofluoran (84; R1 R3, R4 = H, R2 = Cl) is employed, the reaction gives 3′-chloro-6′-cyclohexyl — aminofluoran (85; Ri R*, R4 = H, R2 = C1) together with 3′,6′-dicyclo — hexylaminofluoran (85; Ri R*, R4 = H, R2 = c-C6H11NH). On the other hand, 2′,6′-dichlorofluoran (84; Ri R2, R4 = H, R* = C1) gives solely 2′-chloro-6′-cyclohexylaminofluoran (85; Ri R2, R4 = H, R* = Cl) because of low reactivity of chlorine at 2′-position. 2′-Bromo-6′-chlorofluoran (84; Ri R2, R4 = H, R* = Br) also gives 2′-bromo-6′-cyclohexylaminofluoran (85; Ri R2, R4 = H, R* = Br) in high selectivity.
Table 9 shows melting points of 3 ‘-cyclohexylaminofluorans (85).
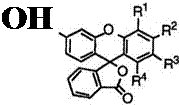 |
Table 9. Melting Points of 3′-Cyclohexylaminofluorans (85)
|
85 |
Ri |
R2 |
R* |
R4 |
О 0 |
|
a |
H |
Cl |
H |
H |
178-181 |
|
b |
H |
H |
Br |
H |
235-237 |
|
c |
H |
H |
Cl |
H |
207-209 |
|
d |
H |
H |
CH3 |
H |
137-140 |
|
e |
H |
CH3 |
Cl |
CH3 |
186-188 |
|
f |
—(CH=CH)2 |
H |
H |
168-170 |
|
|
g |
H |
H |
—(CH=CH)2 — |
222-224 |
Preparation of 3′- Chloro-6′-cydohexylaminoJluoran (85a). A mixture of 3′,6′-dichlorofluoran (0.1 mol), cyclohexylamine hydrochloride (0.15 mol), zinc chloride (0.3 mol), and zinc oxide (0.2 mol) was fused at 190-200 °C for 4 h. After being cooled, the solidified mixture was powdered, heated with 4% hydrochloric acid (1000 ml) to dissolve zinc chloride, and filtered. Then, the filter cake was refluxed with a mixture of toluene (400ml) and 5% aqueous sodium hydroxide (100 ml) for 1 h. The toluene layer was separated, washed with hot water, and concentrated. The residue was refluxed with methanol (200ml) for 1 h. After being cooled, the precipitate was filtered off, washed with methanol, and dried to give 3′-chloro-6′-cyclohexylaminofluoran in 60% yield as an off-white powder, mp 178-181 °C.
 4 сентября, 2015
4 сентября, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 