For the last twenty years, waterborne coatings have been used for the internal spray lacquering of DWI beer/beverage cans, unlike externals. They are used for both steel and aluminium DWI cans. During that time they have progressively replaced solvent borne coatings throughout the world. As long ago as 1979, Robinson of Glidden, now part of ICI Packaging Coatings, presented a paper(17) describing the possibilities of modifying epoxy resins with acrylics to render them water reducible. This technology has also been reviewed by FonenH).
The original waterborne two piece can coating lacquers were based on water dispersible acrylic resins, similar to emulsion polymerisation types. Although simple and flexible in their ease of use within formulations, they quickly proved to be inferior with respect to chemical resistance and flavour impairment problems. Epoxy based films, however, meet the above requirements extremely well. They are in general non-toxic, exhibit relative chemical inertness, give excellent chemical resistance, and can be formulated to give good adhesion and flexibility. Waterborne epoxies have been available to the coatings industry since the early 1960’s and have found extensive use as primers for the automotive and the structural coatings industries. Nevertheless, food contact legislation (FDA in the USA, BGA in Germany etc.) lists materials approved for food contact, and prohibits the use of non-approved materials. This has restricted the use of the waterborne epoxy technology of the automotive industry from being used in can coatings. In order to overcome legislative restrictions, coating technologies have been forced to develop food contact legislation approved waterborne systems.
A waterborne interior two piece can coating lacquer would be formulated using resins similar to those described in Chapter II (VII) (resins 1, 2 and 3). However, the lacquer, no matter which type of resin chemistry is available, must meet certain criteria.
• The coating must be formulated to a suitable viscosity for the chosen application method.
• The rheology (thixotropy, i. e. pseudo-plastic, or dilatant flow) must be considered and adjusted or the coating will not apply well (see later for fuller details).
• The dynamic surface and interfacial tension of the coating must be made compatible with the substrate or substrate wetting problems will occur.
• The adhesion and flexibility of the dry film must be compatible with subsequent processing conditions.
• The resin solids content of the formulation must be sufficient so that the coating will provide the dry film weight required for protection within the can.
• The coating must meet the requirements of the customer’s local and federal environmental legislation.
• Finally but not least, all formulated interior two piece can coatings must be non-toxic, chemically inert, meet organoleptic requirements, and be approved within the current food contact legislation
The resin manufactured for use in can coatings may be the ideal formulation for the processing of the resin, however, the dispersion produced will probably be totally unsuitable for use as a coating lacquer. In the unlikely event the resin dispersion is suitable for use directly as a coating lacquer, one customer’s application technique and equipment specifications will almost certainly be different from another’s. Therefore, the coating formulator will either be required to formulate a single, standard coating to meet all conditions, or a different lacquer for each situation encountered.
The importance of good application cannot be over emphasised as the lacquer is expected to cover the inside of the can in an eighty millisecond pulse of spray for each coat. Polymer structure can affect application. Some aspects of the rheology of waterborne epoxy-acrylic graft copolymers have been reported by Woo et al<19). They make the point that the viscosity of the lacquer under the higher shear conditions as a wet film on the side wall of the can, must be considered. The viscosity of the lacquer under high shear must not be so high as to impair atomisation. Good atomisation is a key to getting good low weight coverage. However, the viscosity of the lacquer at low shear, as a wet film on the wall of the can, must not be so low as to allow ‘drape’ or excessive flow of the wet film as this will cause the build up of small pools of lacquer in the well of the can which can lead to blistering on stoving.
To illustrate this point, in Figure 7-25 a plot of viscosity versus shear rate is shown for three products. System В is a commercial spray lacquer having good low weight coverage and a high blister threshold. System C is an experimental system having a much lower low shear viscosity. This system blisters badly on application. System A, another experimental product, has a higher high shear viscosity and does not atomise correctly and gives poor coverage. In their paper, Woo and Eley(19) showed that the level of benzoyl peroxide initiator used affects the high shear viscosity of the product and the application performance of the lacquer. Blistering is the phenomena which occurs when the solvent cannot escape from the film before the onset of film formation. In escaping, the solvent ‘breaks’ the film causing a ‘blister’. The higher the wet film weight, the greater the tendency for blistering. Blister latitude is a very important requirement for DWI inremal lacquers.
|
Figure 7-25: Viscosity vs Shear Rate for Waterborne Internal spray Lacquers |
A commonly used method of changing the solids/viscosity relationship of these systems is to change the degree of neutralisation, by varying the amount of amine used, Not all of the carboxylic acid groups present in the system need to be neutralised with amine to produce a stable dispersion. The more groups that are neutralised, the greater will be their contribution to the viscosity of the system. So it is possible to increase the viscosity of a dispersion by adding more amine to it, at least up to the point where all the carboxylic acid groups available have been neutralised. The amount of amine present is often measured by titration and can be quoted in milli-equivalents per weight of solid, or volume of coating, and is often referred to as the meq value.
In general the coating viscosity can be adjusted by the addition of a water soluble tertiary amine, hydrophobic solvents and, of course, water. The addition of the tertiary amine will further neutralise a base neutralised epoxy resin (up to 100% neutralisation) and produce an increase in viscosity. The effect of the addition of hydrophobic organic solvents will be to migrate into the dispersed resin, causing a change in the particle size and the size distribution, resulting in a rheological change (increased pseudo-plastic behaviour) in the dispersion. The addition of water reduces the viscosity.
Furthermore, the addition of small quantities of hydroxyl functional crosslinking resins, such as cresol and phenol resoles (phenolics), alkyl melamine and benzoguanamine aminoplast resins, can be used to modify the hardness, adhesion, and flexibility of the original waterborne epoxy. The coalescence and drying function of a waterborne coating is critical to its final performance. The evaporation rate of the mobile phase can be modified, and the film given a longer open time, thereby avoiding water entrapment and blistering during drying.
The careful selection and addition of organic solvents (hydrophilic, hydrophobic, resin compatible and resin incompatible) to the coatings will improve the substrate wetting and modify the coalescence of the applied coating during high temperature curing. The use of water soluble polymers will modify the application characteristics of the wet coating over the substrate towards that of a solvent system. Surfactants, such as polyethylene oxide-polypropylene oxide, alkyl phenol ethoxylates, sorbitol modified dioctyl sulfosuccintes, alkylated alkynols, can be used to modify the dynamic surface tension towards that of the substrate, and the interfacial tension between the stationary and mobile phases of the dispersion — provided, of course, that they comply with food contact legislation, if they are to remain in the film. The reduction in surface tension can dramatically improve the application, wetting and dry film appearance of the coating. However, unless the surfactant is bonded within the coating, or is driven off during high temperature curing, the material can remain free within the dry film, generating potential organoleptic and chemical resistance problems.
Typically, the organic solvents used in an internal lacquer are generally of the following types ; alkyl alcohol’s, aliphatic and aromatic ketones, glycol ethers, aliphatic and aromatic solvents. The solvent choice must be based upon the solvent properties and on consideration of the lacquer application technique.
The commonly preferred solvents of the past twenty years have been based around the lower homologues of ethylene glycol ether. Unfortunately, from the formulator’s viewpoint, this type of solvent is being phased out of coatings formulations by pressure from environmental and HSE legislation (e. g. US federal HAP’s legislation). The alternatives available exploit the higher homologues of ethylene glycol ether and, of course, the homologues of propylene glycol ethers. Although the ester variations of the glycol ethers are available to the formulator, great care must be taken to ascertain the hydrolysis stability of the ester being considered. Careful study of the hydrophobic/hydrophilic tendency of the ester will indicate whether water compatibility would propagate an undesired (delayed) hydrolysis reaction to the carboxylic acid form of the alkyl ester. The hydrolysis reaction can take from a few days to a few weeks, however the reaction can be accelerated in the presence of other acid groups, and certain surfactants. It should also be noted that glycol ether esters are not the only solvents capable of hydrolysis, certain ketones will exhibit similar delayed hydrolysis, as will waterborne epoxies but over a period of up to six months as opposed to weeks.
The following formulation is typical of type used as a spray applied beverage can coating lacquer.
(i) DWI Internal Spray Lacquer
|
FORMULATION 7-27
|
Viscosity: 24 secs Ford #4 Cup @25°C
nvc 24% 200°C for 3 minutes
(1) Texanol is a commercial name for 2,2,4-trimethyl-1,3-pentanediolmonoisobutyrate
(2) Trademark of Dow Solvents
(3) Ex BIP
It should be noted that DMAE would frequently be used instead of TEA (triethylamine). Epoxy resin 3 is the epoxy graft acrylic resin, the preparation of which was described in Chapter II (VII.3)
The Texanol and Dowanol PM are added slowly to the epoxy dispersion under agitation and are mixed together for 20 minutes. The crosslinker (BE 3747) is a fully methylated melamine (HMMM) of low viscosity, however without the presence of a coupling solvent (or co-solvent), such as butanol, it is not water dispersible. The acid functionality of the epoxy acrylic will act as an internal catalyst, because fully methylated melamines require acid catalysis for optimum properties to be achieved. The crosslinker is added to the dispersion under maximum agitation, so that it enters the dispersion at the centre of the agitator’s vortex. The mixture is agitated continuously for a further 40 minutes. The dispersion is then adjusted to the final solids, and viscosity by the careful addition of DIW, and tertiary amine to reach the solids and viscosity required.
The above formulation can be airless spray applied to a two piece beverage can giving a flexible, protective coating with excellent adhesion to aluminium or ETP. If required, the coating viscosity/solids relationship can be modified by carefully balancing the DIW and tertiary amine (TEA) addition, to give a lower solids, higher viscosity, or higher solids, lower viscosity coating.
 11 октября, 2015
11 октября, 2015  Malyar
Malyar 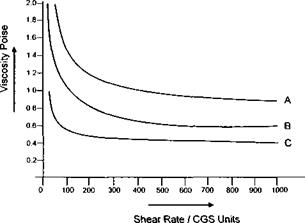
 Опубликовано в рубрике
Опубликовано в рубрике 