External coatings for the DWI 2 piece beer and beverage can market are moving away from solvent to water based systems. However the metal used to construct the can may influence whether the external coating is solvent based or waterborne. A further complication in Europe is that both steel (ETP) and aluminium DWI cans are manufactured (about a 50/50 split) unlike the USA where it is virtually 100% aluminium. Steel is much harder to coat than aluminium, particularly for waterborne systems. Because a coating works on aluminium, it does not mean that it will perform on steel. Different film weights and stoving conditions may also be encountered between the two different types of substrate cans.
DWI external coatings are split between acrylic and polyester systems, both of which contain carboxy functionality, neutralised with an amine such as dimethylethanolamine. Current developments in DWI basecoat technology are towards hybrid acrylic polyester water systems. The acrylic portion provides good pigment dispersion properties, whilst the polyester will give improved performance during the spin necking operation.
The acrylic resin used will be similar in composition to the solvent based compositions. The comonomers which can be used are relatively restricted due to the high demands of performance. The major difference will be in the ‘hard’ comonomer. Whilst styrene is lower cost it will not survive extreme necking operations, thus methyl methacrylate would normally be used for systems intended for all but the mildest of necking operations. Butyl or ethyl acrylate would be the soft comonomers. Due to odour and toxicity, butyl is the preferred acrylate. However, performance requirements may dictate otherwise.
One major difference between the use of waterborne acrylic (or polyester) systems on the inside of the can and their use on the outside, is that whilst water dispersed systems have desirable application characteristics for spray application, they are not so good at giving good flow in films applied by a roller coater. It is therefore more likely that the polymer binder system employed in the manufacture of an external white coating will be soluble
|
442 |
VII — Waterborne Acrylic Systems for Industrial Applications |
|
in the water/organic solvent used as the continuous phase. The resin system must then be formulated for optimum pigment wetting, cure response, adhesion, flexibility and scuff resistance. Some of the monomers used in acrylics for waterborne white coatings are shown in Figure 7-23. |
|
|
Acrylic Acid |
Methacrylic Acid |
|
/H H2C=C’ /0 c 0 —H |
/CH3 H2C = c’ 0 c o—H |
|
Esters of Acrylic Acid |
Esters of Methacrylic Acid |
|
/H H2C= c’ 0 c 0—R |
/CH3 н2с = сГ о 4< o—R |
Where;
R is — CH3, — CH2 — CH3, CH2 — CH2 — CH2 — CH3, — CH2 — CH2 — OH, — CH2 — CH — CH3 Methyl Ethyl n-Butyl Hydroxyl Ethyl 2-Hydroxy Propyl
Figure 7-23: Some Acrylic Monomers which can be Used for Waterborne External DWI
Coatings.
Frequently, hydroxyl functional monomers are used as part of the copolymer with the hydroxy group providing the site for crosslinking with, for example, an amino crosslinker, when the coating is stoved. Two hydroxy functional types have been shown. Hydroxyl ethyl has a terminal (primary) hydroxyl group which is more reactive than the secondary hydroxy group on the hydroxy propyl chain. It is thus possible to get more rapid cure response by incorporation of hydroxy ethyl rather than hydroxy propyl functionality in the acrylic copolymer. This, however, may not always be advantageous as frequently rapid cure can lead to poor adhesion.
Another means of introducing functional groups into the acrylic resin is by use of alkylated methylol acrylamide, shown in Figure 7-24. As well as having the potential for crosslinking with amino crosslinkers, the butylated methylol groups are capable of crosslinking with themselves, thus so called self-curing systems can thus be formulated.
Another very useful formulating tool is the concept of polymer glass transition temperature or Tg. The glass transition temperature is the temperature below which a polymer would change into a brittle glassy substance. Copolymers made by using varying amounts of different monomers can thus be formulated with a wide range of glass transition temperatures. Monomers such as methyl methacrylate would be included to provide toughness and scratch resistance. Whereas monomers with Tg’s below room temperature, such as butyl acrylate, would provide better flexibility and wetting and, in this case, being more hydrophobic it will be less water soluble.
Of course, the molecular weight of the polymer will also be very influential on properties. A relatively low molecular weight polymer relying on a high degree of cure with crosslinking will not be as flexible as a higher molecular weight polymer with a lower degree of crosslinking. Once again, it can be seen that polymers need to be designed for specific applications and conditions of use, in order for optimum coating performance to be achieved.
Nearly all the DWI formulations are proprietary, particularly those for steel cans. Thus, only a generalised water based coating formulation for aluminium DWI cans can be considered.
(i) White Water Reducible Acrylic for DWI Aluminium Cans
|
Dispersion Stage rutile titanium dioxide |
16.16 |
|
carboxy functional flexible*1′ acrylic (65% in Dowanol PM) |
15.53 |
|
dimethylethanolamine Byk P1045*2′ |
10.80 0.37 |
|
Dowanol PM *3′ |
1.20 |
|
butyl carbitol*4′ |
1.20 |
|
water |
10.80 |
|
Let Down Stage Premix: carboxy functional flexible*1′ acrylic |
9.85 |
|
(65% in Dowanol PM) |
|
|
dimethylethanolamine HMMM*5′ |
0.36 3.50 |
|
Dowanol*3′ |
0.60 |
|
water |
23.33 |
|
dinonyl napthalene*6′ disulphonic acid |
0.30 |
|
water |
6.00 |
|
Total |
100.00 |
|
FORMULATION 7-26 |
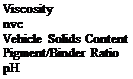 |
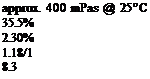 |
Cure to peak metal temperature (pmt) 190°C
(1) Water reducible acrylic ( acid value ca 100 mg KOH/g )
(2> Byk Chemie
*3) Dow Chemicals
!4) Union Carbide
l5> Cymol 303 Am Cyanol
<6> Nacure 155
In this formulation, DMAE is used as the neutralising amine. The solvent system is matched for the application conditions and stoving schedule. The crosslinking system is melamine which is acid catalysed by dinonyl napthalene disulphonic acid. As a general
rule, melamines give the best compromise of cost, hardness, flexibility, durability and resistance properties.
Different companies and different geographic areas may or may not use a varnish. The water based varnish would be based on a similar resin system to the base coat. If no varnish is used, the system is frequently referred to as ’NOVAR’. As described in the solvent based metal decorating section, the final coat must give the can the required degree of mobility for the filling lines. This is normally achieved by the inclusion of wax or slip aids or both. Silicones would not be used, due to the risk they may pose to the incomplete coverage of the internal lacquer which is applied last. Silicones can cause cissing and other serious dewetting problems. Failure of an internal lacquer to completely protect the surface of the can and its contents from the can will result in large claims. If a NOVAR system is used, then the basecoat must contain the mobility additives, which must be suitable for water based systems. For varnished systems, the varnish contains them. In many instances, coating formulations are modified for particular DWI lines. Each line will vary and the coating may need modification before it will run satisfactorily, trouble free, day in day out.
 11 октября, 2015
11 октября, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 