Latex-type binders account for a large volume of waterbased coatings. Film formation in a latex paint involves the coalescence or fusion of discrete polymer particles of high MW polymers. When the film is applied, the polymer particles are pushed against each other under the capillary forces generated due to progressive evaporation of water. This results in coalescence at the particle boundaries, with interdiffusion of polymer chain across the boundaries (Figure 5.17).
For this phenomenon to occur successfully, the molecules within the polymer particles must have sufficient mobility to facilitate
|
|
coalescence and allow the formation of a coninuous, integral film. This is most successful if the polymer has a sufficiently low Tg. The resulting films in such cases will be too soft to meet some of the resistance properties required by paint films. In order to address this problem, the Tg of a latex polymer can be reduced temporarily by the incorporation of high boiling solvents, known as coalescing agents, that will eventually evaporate, leaving a hard film due to the high Tg of the original latex polymer. Thus, coalescing agents are additives that are added to provide temporary plasticization of the latex particles, which is necessary for efficient coalescence and hence film formation.
For each latex, there is a characteristic limiting temperature above which the polymer particles are soft enough to coalescence completely under the influence of capillary forces. This temperature is known as the minimum film-forming temperature. The coalescing agents help in lowering of this temperature for a given latex and facilitate coalescence and the film formation process of latex paints, even at temperatures lower than the minimum film-forming temperature (MFFT) of the latex.
A typical coalescing agent is an organic solvent that exhibits most of the following characteristics:
• Migrates into and swells the polymer particle
• Has no effect on the stability of latex
• Has minimum solubility in water so that it preferentially dissolves in the polymer
• Evaporates quickly after film formation
• Has low odor.
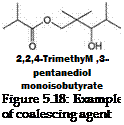 Glycols, glycol ethers and their acetates, and ester alcohols have been widely used as coalescing solvents. Many of the coalescing agents are VOCs; the recent trend is to use non-VOC type coalescing agents. One of the most commonly used coalescing agents is
Glycols, glycol ethers and their acetates, and ester alcohols have been widely used as coalescing solvents. Many of the coalescing agents are VOCs; the recent trend is to use non-VOC type coalescing agents. One of the most commonly used coalescing agents is
from the class of ester alcohols (Figure 5.18). In addition to facilitating film formation, coalescing agents can also influence other characteristics of coatings such as flow and leveling, wet-edge time and rheology.
 17 января, 2016
17 января, 2016  Pokraskin
Pokraskin 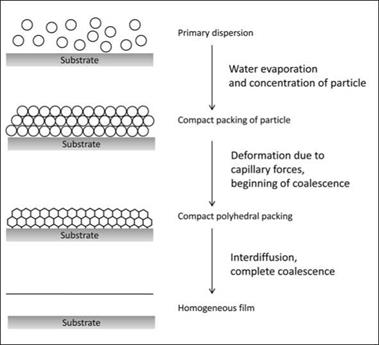
 Опубликовано в рубрике
Опубликовано в рубрике 