Silicone rubber, polytetrafluoroethylene (PTFE), Acetal and the polyolefin plastics (polypropylene, polyethylene) are always a challenge to the adhesive engineer due to the low surface energy of these materials. Whilst the detailed consideration of surface tension is more in the province of the physicist than the engineer, wetting (the establishment of contact) plays a significant role in adhesion.
Surface tension causes many liquids to behave as an elastic sheet and allows insects, such as the water boatman, to walk on water (Figure 6.1). It also allows small objects, even metal ones such as needles and razor blades, to float on the surface of water and it is the cause of capillary action.
For good wetting and therefore good adhesion, the adhesive must be capable of spreading over the solid surface displacing air and any other surface contaminants that may be present. The scientific study of interfacial properties has developed measurement and analytical techniques that give a detailed analysis of components that determine wetting equilibria and surface/interfacial energy.
|
Figure 6.1 The surface tension allows the water boatman to ‘walk’ on water |
Surface tension has the dimension of force per unit length or of energy per unit area. The two are equivalent — but when referring to energy per unit of area most engineers use the term surface energy, which is a more general term in the sense that it applies also to solids and not just liquids.
For many years surface tension was measured in dynes/cm and many engineers still use this unit today. The modern SI unit however is mN/m (milli-Newtons per metre) and is the same as dynes/cm.
Since no liquid can exist in a perfect vacuum for very long, the surface of any liquid is an interface between that liquid and some other medium. The top surface of a pond, for example, is an interface between the pond water and the air. Surface tension, then, is not a property of the liquid alone, but a property of the liquid’s interface with another medium.
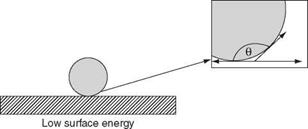
Young [1] developed the relationship between the contact angle and the three interfacial tension points that describe a sessile drop, Equation 6.1.
Ysv = Ysi + Yiv cose (6.1)
where Ysv is the solid/vapour point, ysl is the solid/liquid point, yv is the liquid/vapour point and 0 is the contact angle (Figure 6.2).
|
|
|
High surface energy Figure 6.2 Low contact angles favour better wetting |
For the common sessile or pendant drop shape the Laplace equation describes the relationship of the two radii of the elliptical sessile drop with the pressure across the surface and the surface tension, Equation 6.2.
AP=+R) (a2>
where AP is the pressure, о is the surface tension and R1 and R2 are the principal radii of curvature.
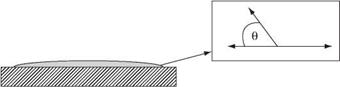
For an adhesive to ‘wet’ a surface, it requires a lower surface tension than the surface energy of the solid. If this condition is not met, the liquid does not spread across the surface but forms spherical droplets on the surface. Water has a relatively high surface tension (70 mN/m) and so on a highly polished car bonnet, the water will form droplets (Figure 6.3) because the waxed surface of the metal bonnet will have a lower surface energy than the water and so prevents wetting.
Wetting of plastic surfaces is much more complex than wetting clean metal surfaces. Plastics and adhesives are both polymeric materials and thus have similar physical properties, including wetting tensions. Plastic-bonded joints do not have the large difference between the critical surface tension of the substrate and that of the adhesive,
|
Figure 6.3 Water forming droplets on a polished car bonnet [2] |
which ensures wetting for metals. In addition, many plastics have notoriously low critical wetting tensions. Polyethylene (PE) and polypropylene (PP), with critical surface tensions of 31 and 29 mN/m respectively, present serious wetting challenges for most adhesives. The surface tension for an ethyl cyanoacrylate is 33 mN/m and so the surface energy of the solid must be greater than 33 mN/m to achieve good wetting. Other plastics such as polystyrene and polyvinyl chloride (PVC) have higher critical surface tensions and present less of a problem.
Table 6.1 shows some surface energy values for a range of materials and it can be seen that PTFE has a surface energy of 18 mN/m and therefore cannot be bonded without surface pre-treatment. PVC, however, has a surface energy of about 38 mN/m and can therefore be bonded.
Most industrial adhesives (e. g., cyanoacrylate, epoxy, polyurethane, room-temperature- vulcanising silicone and most acrylic adhesives) do not adhere to PP and PE. Indeed these adhesives are often packaged in PP or PE bottles so that the adhesive itself can be dispensed without sticking to the bottle.
|
Table 6.1 Surface tension values for some plastics |
|
|
Material |
Surface tension (mN/m) |
|
PTFE |
18 |
|
Acetal |
22 |
|
Polypropylene |
29 |
|
Polyethylene |
31 |
|
Polystyrene |
35-37 |
|
Polymethylmethacrylate (acrylic) |
39 |
|
PVC |
39 |
|
Polyethylene terephthalate |
41-47 |
|
Polycarbonate |
46 |
|
Nylon 6 |
46 |
Polyolefins and fluoropolymers are also difficult to bond for other reasons:
• Low porosity — there is no opportunity for the adhesive to penetrate into the plastic and give mechanical interlocking.
• No functional groups — polyolefins are comprised entirely of carbon and hydrogen atoms and are very non-polar polymers. Most adhesives contain oxygen, nitrogen
and other electron-rich atoms and are polar materials, and if (like polyolefins) the carbon and hydrogen bonds are very unreactive, there is no opportunity for the adhesive to form chemical bonds.
• Surface weaknesses — Some plastics have weak boundary layers due to the low tensile strengths between some of the molecules at the surface of the plastic.
• Mould release agents can also be the cause of low adhesion if they are silicone or PTFE based and are transferred across from the mould tool.
 9 ноября, 2015
9 ноября, 2015  Pokraskin
Pokraskin 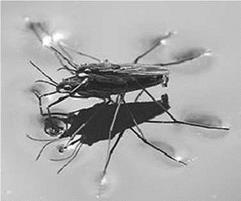

 Опубликовано в рубрике
Опубликовано в рубрике 