Some physical and chemical properties of ZnS and BaSO4 are given in Table 2.10.
|
Tab. 2.10: Properties of the components of zinc sulfide pigments.
’Component oflithopone. |
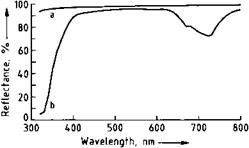
A white pigment must not absorb light in the visible region (wavelength 400800 nm), but should disperse incident radiation in this region as completely as possible. The spectral reflectance curves of zinc sulfide and barium sulfate (Figure 2.6) fulfill these conditions to a large extent. The absorption maximum for ZnS at ca. 700 nm is a result of lattice stabilization with cobalt ions, whose function is explained in Section 2.2.2.2. The absorption edge in the UV-A region is responsible for the bluish-white tinge of zinc sulfide. Depending on the production process, zinc sulfide has a sphalerite or wurtzite lattice type.
The refractive index n of ZnS, which determines its scattering properties, is 2.37 and is much greater than that of plastics and binders (n = 1.5-1.6). Spheroidal ZnS particles have their maximum scattering power at a diameter of 294 nm. Barium sulfate does not directly contribute to the light scattering due to its relatively low refractive index (n = 1.64), but acts as an extender, and increases the scattering efficiency of the ZnS.
The barium sulfate in lithopone can be identified thermoanalytically by a reversible endothermic transformation at 1150 °C. Both Sachtolith and lithopone are thermally stable up to ca. 550 °C in the presence of air. Due to their low Mohs hardness, they are less abrasive than other white pigments. Barium sulfate is practically inert toward acids, bases, and organic solvents. Zinc sulfide is stable in aqueous media
Fig. 2.6 Spectral reflectance curves of barium sulfate and zinc sulfide. a) Barium sulfate, b) Zinc sulfide (Codoped)
 between pH 4 and 10, and is largely inert toward organic media. In the presence of water and oxygen, it can be oxidatively decomposed by the action of UV radiation.
between pH 4 and 10, and is largely inert toward organic media. In the presence of water and oxygen, it can be oxidatively decomposed by the action of UV radiation.
2.2.2
 6 октября, 2015
6 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 