The reaction of equimolar quantities of ZnSO4 and BaS produces a white, water — insoluble coprecipitate with the theoretical composition 29.4 wt.% ZnS and 70.6 wt.% BaSO4:
ZnSO4 + BaS ^ZnS + BaSO4
By using a different molar ratio, this composition can be changed; for example, precipitation according to the following equation gives a product containing 62.5 wt.% ZnS and 37.5 wt.% BaSO4:
ZnSO4 + 3 ZnCl2+4 BaS ^4 ZnS + BaSO4 + 3 BaCl2
Figure 2.7 is a flow diagram of lithopone production. The solutions of zinc salts contain impurities (e. g., salts of iron, nickel, chromium, manganese, silver, cadmium) that depend on their origins. The main sources of zinc sulfate solutions are zinc electrolyses and the reprocessing of zinc scrap and zinc oxide. The first stage of purification consists of chlorination. Iron and manganese are precipitated as oxide-hydroxides,
and cobalt, nickel, and cadmium as hydroxides. The solutions are then mixed with zinc dust at 80 °C. All the elements more noble than zinc (cadmium, indium, thallium, nickel, cobalt, lead, iron, copper, and silver) are almost completely precipitated, while zinc goes into solution. The metal slime is filtered off and recycled. A small quantity of a water-soluble cobalt salt is added to the purified zinc salt solution. The cobalt (0.02-0.5%) becomes incorporated into the ZnS lattice during subsequent calcination to stabilize the final product against light. Zinc sulfide, that is not treated in this way becomes gray in sunlight.
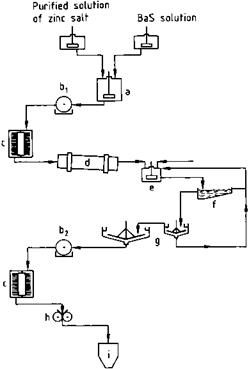 Fig. 2.7 Flow diagram for lithopone production. a) Precipitation vessel; b) Rotary filter, c) Turbo dryer; d) Rotary kiln; e) Chilling vessel; f) Rake classifier; g) Thickener; h) Grinder; i) Silo.
Fig. 2.7 Flow diagram for lithopone production. a) Precipitation vessel; b) Rotary filter, c) Turbo dryer; d) Rotary kiln; e) Chilling vessel; f) Rake classifier; g) Thickener; h) Grinder; i) Silo.
The barium sulfide solution is produced by dissolving fused barium sulfide in water. The barium sulfide is obtained by reducing an intimate mixture of crushed barite (ca. 1 cm lumps) with petroleum coke according to the following equation in a directly heated rotary kiln at 1200-1300 °C:
BaSO4 + 2C ——BaS + 2CO2
The warm solution (60 °C) containing ca. 200 g L-1 barium sulfide is filtered and immediately pumped to the precipitation stage. Further purification is not necessary. Unreacted gangue and heavy metals are collected as insoluble sulfides in the filter cake. The almost clear solution can be stored only for a short period. Longer storage leads to undesirable polysulfide formation.
The zinc salt and BaS solutions are mixed thoroughly under controlled conditions (vessel geometry, temperature, pH, salt concentration, and stirring speed, see (a) in Figure 2.7). The precipitated “raw lithopone” does not possess pigment properties.
It is filtered off (b1) and dried (c); ca. 2 cm lumps of the material are calcined in a rotary kiln (d), then directly heated with natural gas at 650-700 °C. Crystal growth is controlled by traces of sodium, potassium and magnesium salts. The temperature profile and residence time in the kiln are controlled to obtain ZnS with an optimum particle size of ca. 300 nm.
The hot product from the kiln is quenched in water (e), passed via classifiers and hydroseparators (f) into thickeners (g), filtered on rotary filters (b2), and washed until salt-free. The dried product is ground in high-intensity mills (g) and may undergo organic treatment depending on the application.
Figure 2.8 shows a scanning electron micrograph of lithopone. The ZnS and BaSO4 particles can be distinguished by means of their size. The average particle diameter of BaSO4 is 1 pm.
 Fig. 2.8 Scanning electron micrograph oflithopone. The larger particles are barium sulfate (mean size 1.0 pm) and the smaller particles are zinc sulfide (mean particle size 0.3 pm).
Fig. 2.8 Scanning electron micrograph oflithopone. The larger particles are barium sulfate (mean size 1.0 pm) and the smaller particles are zinc sulfide (mean particle size 0.3 pm).
 8 октября, 2015
8 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 