In the case of charge transfer, the optical transition takes place between different kinds of orbitals or between electronic states of different ions. In these cases, too, the width and position of the emission bands depend on the chemical environment.
A very well known example is CaWO4, used for decades for the detection of X-rays, which shows luminescence originating from the (WO4)2- group (Figure 5.49). The transition involves charge transfer from oxygen ions to empty d-levels of the tungsten ion. Here, the chemical bonding character changes very strongly, reflected in a very broad emission spectrum. As in this material no intentional dopant is introduced, it is also called self-activated.
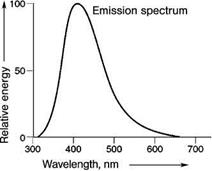 Fig. 5.49 Emission spectrum of CaWO4.
Fig. 5.49 Emission spectrum of CaWO4.
276I 5 Specialty Pigments
5.5.3.3
 19 января, 2016
19 января, 2016  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 