Dehydrothiotoluidinesulfonic acid—N ^
^N
"Dehydrothiotoluidinesulfonic acid—NH
An amount of dehydrothiotoluidinesulfonic acid, equivalent to 14 grams of sodium nitrite, is dissolved in 25 grams of 30 per cent sodium hydroxide. Half of this solution is acidified with 25 cc. concentrated hydrochloric acid and diazotized at 10°C. in the course of 2 hours with 7 grams of sodium nitrite. The resulting orange yellow diazo compound is mixed with the other half of the original sulfonic acid solution to which has been added 25 grams of soda ash in a small amount of water and 25 cc. concentrated ammonia. The coupling temperature should be
4- 5°, and it is desirable to have the solutions as concentrated as possible. After 2 hours, the mixture is warmed to 30° and allowed to stand overnight. It is then heated to 80°, and the dye is salted out by adding 20 per cent of salt by volume. The yield of concentrated dye is about 85 grams.
Thiazole yellow (Clayton yellow, mimosa, etc.) is, in contrast to chloramine yellow, the least fast yellow of the entire dye industry and it is really astonishing that such an inferior dye is used at all. It has, how
ever, high color purity and strength, and is used for inexpensive dyed textile materials.
Thiazole yellow is changed by sodium hydroxide from a pure yellow to a bright red, and it can be used, therefore, as a reagent to test for alkali (thiazole paper).
Sulfonation of pritnuline-like color bases by the “baking” process yields sulfonic acids whose azo dye derivatives are more fast to light than those from sulfonic acids prepared in the ordinary way. It is assumed that in the “baking” process, the sulfo group enters ortho to the amino group, and that this increases the light fastness. This same principle was mentioned in connection with the pyrazolone dyes.
Technical Observations: Primuline fusions are handled in vessels heated in
oil baths and having condensers supplied with warm water so that the p-toluidine which sublimes does not stop up the tubes. The hydrogen sulfide is collected in sodium hydroxide and used in reductions. Originally, the hydrogen sulfide was burned to heat the vessels, a procedure which is irrational in every respect and a nuisance for the neighborhood. The alcohol extraction is carried out in iron vessels having a fine sieve and filter at the bottom, and the alcohol is distilled back into the vessel just as in a Soxhlet extractor. After the alcohol is evaporated from the extract, the residue is heated to 240° until no more p toluidine is being recovered.
Products analogous to chloramine yellow and thiazole yellow can be prepared from primuline, but these dyes give much muddier colors and are redder and weaker so they have found little favor.
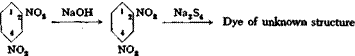 |
Sulfur Black T from Dinitrochlorobenzene
In a glass or iron vessel (such as shown in Fig. 36), 120 cc. water and 70 grams of 2,4-dinitrochlorobenzene are heated to 90°C. with stirring, and 80 grams of 35 per cent sodium hydroxide is added over a period of 2 hours. The reaction mixture should not be strongly alkaline at any time. Heating is continued until a test sample dissolves in water to give a clear solution; more sodium hydroxide is added if necessary. The suspension of sodium dinitrophenolate is cooled to 45° and mixed with a solution of 50 grams of sulfur in 125 grams of water containing 125 grams of crystalline sodium sulfide. The temperature is raised to 60° and the volume brought up to 600 cc. Then the temperature is carefully raised to 80° (water bath) and, in the course of 2.5 hours, to 105° (oil bath). The mixture is then boiled under reflux without stirring for 30 hours, and thereafter diluted with 600 cc. water. Air is passed into the reaction mixture at 60° until the dye is precipitated. It
is filtered off and dried at 70°. The yield is about 70 grams. In practice, the dye is used in boiling solution containing 1 part of dye, 4 parts of sodium sulfide (crystalline), and salt equal in weight to the weight of the cotton.
Technical Observations. Sulphur black T is the most widely used sulphur black, and is unsurpassed for washing and light fastness. It is manufactured starting with batches of 500 to 1500 kilograms of dinitrochlorobenzene. The melting kettles hold up to 12,000 liters and the oxidation vessels up to 30,000 liters. With such large batches, it is not necessary to supply heat, the heat of reaction being sufficient. The kettles are of cast iron and are corroded rapidly. The mother liquor yields sodium thiosulfate, which finds use in the photographic and textile fields. Part of it is used in the dye plant in making methylene blue. The price of sulphur black T, for about a 35 per cent product, is 80 to 90 “rappen,” and its manufacture is successful only in those plants where all of the by-products are recovered. Furthermore, manufacturers who do not make their own chlorobenzene and dinitrochlorobenzene are unable to compete.
The dinitrochlorobenzene used in the preparation of sulphur black T should be free from the 2,6 isomer.
 25 декабря, 2015
25 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 