Sulfonation of /З-naphthol under various conditions of temperature and sulfuric acid concentration gives rise to a whole series of mono — and
polysulfonic acids. Only a few of the typical reactions will be described in detail here in order to give the beginner a picture of this field of intermediates.
The first product formed by the actio^ of concentrated sulfuric acid on /ї-naphthol is 2-naphthol-l-sulfonic acid (page 199). This compound, however, is very unstable and rearranges, even in the cold, in the presence of excess sulfuric acid, to form 2-naphthol-8-sulfonic acid (Bayer acid, croceine acid), and this in turn rearranges, only partially in the cold but completely at higher temperatures, into 2-naphthol-6- sulfonic acid (Schaeffer acid).
When an excess of acid is used, there are always formed also some
2- naphthol-3,6-disuIfonic acid and 2-naphthol-6,8-disulfonic acid. These disulfonic acids usually react with diazonium salts to yield, respectively, red and yellow dyes, and are therefore known commonly as R acid (R—rot) and G acid (G~gelb). R and G acids are the main sulfonation products if a large enough excess of sulfuric acid is used, relatively more of the R acid being formed at low temperatures and more G acid at higher temperatures. Under very vigorous conditions (oleum at elevated temperatures), both disulfonic acids are converted to 2-naphthol-
3,6,8- trisulfonic acid. All of these acids are important starting materials in the azo dye industry. In most cases, it is practically impossible to prepare any one of them separately; it is necessary, as a rule, to separate them from mixtures.
|
|
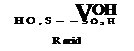 |
|
|
|
![]()
![]()
HO, S-»
2| OH
j—so, H
Sulfonation reactions, especially those carried out at low temperatures, often form, in addition to the above acids, appreciable quantities of 2-naphthol-l,6-disulfonic acid and 2-naphthol-l,3,6-trisulfonic acid. Since these products have no technical value, the sulfo group in the 1 position is split off by diluting the sulfonation mixtures with water and heating for 0.5 to 1 hour at 90-100°.
Separation of the individual sulfonic acids from a reaction mixture is based on the different solubilities of their alkali salts. The three acids which are technically the most important and also which are most frequently formed simultaneously are the 2,6-monosulfonic acid (Schaeffer acid), and the 2,3,6-, and 2,6,8-disulfonic acids (R and G acids). Of these, the first gives a sodium salt having the lowest solubility in cold water, and the presence of salt has little effect on its relatively low solubility. To the contrary, the sodium salt of R acid is much more soluble in pure water, but is largely precipitated by a relatively small amount of salt. The sodium salt of G acid is easily soluble in highly concentrated salt solutions; on the other hand, its potassium salt is only moderately soluble in cold water and still less soluble in potassium chloride solutions. The foregoing statements apply to the neutral salts in which all of the sulfo groups are neutralized but the hydroxyl group is free. Notwithstanding statements in the literature to the contrary, the salts which separate from neutral or acid solution are always these neutral salts and never the acid salts in which free sulfo groups are still present. In alkaline solution, however, basic salts are formed, in which the hydroxyl group is also neutralized. These basic salts are more easily soluble than the neutral salts and are not suitable for isolation. Separation of mixtures, therefore, is always carried out in neutral or acidic solution.
In practice, the neutral or slightly acid solution of the sodium salts, containing little or no sodium chloride, is evaporated to a definite volume which depends on the composition of the reaction mixture and which is selected so that on cooling only the sodium 2-naphthol-6-sulfonate crystallizes out, but this as completely as possible. The precipitate is removed, and, to the filtrate, usually after further evaporation, is added enough salt to make it an 18 to 20 per cent salt solution. R salt then precipitates when the solution is cooled. Potassium chloride is then added to the mother liquor to precipitate the potassium salt of G acid.
In order to obtain really pure products from a given reaction mixture, not only is it necessary to have exactly the correct concentration and quantity of salt, but the filtration and washing of the separate precipitates must be done with exacting care in order to remove the
mother liquor as completely as possible with a minimum of wash liquid.
It should be emphasized that /З-naphthol should always be finely pulverized before use. If this precaution is not taken, part of the naph — thol is sulfonated but the large lumps floating around in the mixture are not attacked and the results are completely unsatisfactory. This is also the case if the reaction mixture is merely allowed to stand and is not stirred continuously. The apparatus used is the same as that used for the preparation of naphthalene-/?-sulfonic acid (Fig. 19).
 22 октября, 2015
22 октября, 2015  Pokraskin
Pokraskin 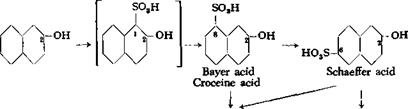
 Опубликовано в рубрике
Опубликовано в рубрике 