The reduction method described above, using iron in the presence of a small amount of acid, was originated by Bechamp and Brimmeyr and is the most widely used technical reduction method. In general it runs very smoothly, provided that the following conditions are adhered to:
1. The reaction vessel must be metal. Only wrought iron is used industrially, but in the laboratory copper vessels can be used to advantage. Glass, porcelain, and enameled vessels are not usable. However, if it is desired to use such vessels in the laboratory, a small quantity of a soluble copper salt must be added. Then metallic copper deposits on the surface of the iron and this appreciably increases the reactivity of the iron.
2. The iron used in the reduction must be cast iron. Borings or shavings are washed with an organic solvent to remove grease, then they are ground to a fine powder in a ball mill. For sensitive reactions, it is recommended that the iron powder be screened to remove the larger particles. Wrought iron waste, nails, and the like are not usable.
3. The stirrer must extend to the bottom of the reaction vessel and run rapidly enough to stir up the iron powder despite its weight.
4. Precautions should be taken that the intermediate phenylhydroxylamine (see reaction above) be reduced as rapidly as possible. When working with laboratory quantities it is advisable, therefore, to add the nitro compound slowly to the reaction mixture so that each portion is reduced to the amine stage as rapidly as possible. The reverse procedure is often used in large scale operations where longer reaction times are undesirable.
The reduction of nitrobenzene proceeds through the intermediate steps of nitrosobenzene and phenylhydroxylamine, as shown in the reaction above. Although nitrosobenzene is reduced so rapidly that it can scarcely be isolated, phenylhydroxylamine can be obtained in good yield as the main product under suitable reduction conditions. Phenylhydroxylamine is also easily reduced to aniline, but it undergoes other transformations very easily too. Thus, it condenses extremely easily with nitrosobenzene to form azoxybenzene, especially in the presence of alkali (see preparation of benzidine, page 124), or reacts with itself to form azobenzene (yellow coloration!). These products, on continued reduction, yield hydrazobenzene and finally aniline; the reductions, however, are often much slower and less smooth than the direct reduction of the hydroxylamine to aniline. In order to avoid these side reactions the reduction mixture must be kept acid at all times, even if only weakly so. On the other hand, phenylhydroxylamine can be rearranged to p-aminophenol by dilute sulfuric acid, or converted to halogenated derivatives by the action of halogen acids. Hence, the surest method for obtaining smooth reduction is to use acetic acid in the reaction. Equally good results are obtained in many cases by the use of hydrochloric or sulfuric acid in sufficient dilution. The nature of the acid and the optimum amount must be determined for each individual case.
It is frequently advantageous to use somewhat more acid in the laboratory than is used in technical operations, perhaps up to one-half equivalent per mole of nitro compound. If too much acid is used, however, too much of the iron goes into solution and then, when the reaction mixture is made alkaline, a voluminous precipitate of iron hydroxide is formed, making filtration very difficult.
The B^champ — Brimmeyr reduction is usually carried out at the boiling temperature, but with especially sensitive substances (for example, p-nitrosodimethylaniline) a temperature of 80° is used. The reaction generates heat and the externally applied heat is regulated so that too vigorous boiling does not occur. Severe foaming occurs in many of the reductions. In these cases, the reaction vessel is filled no more than half full, and the nitro compound is added very slowly and carefully; if the mixture still threatens to foam over, the foam is broken by being sprinkled with a few drops of cold water.
Working up of the reaction mixture varies according to the nature of the reduction product. If the product is volatile with steam, the reaction mixture is made alkaline and the product separated by steam distillation. In other cases, the reaction mixture is filtered with suction after having been made alkaline. Then water or alkali soluble amino compounds, especially sulfonic and carboxylic acids, are found in the filtrate, from which they may be isolated by acidifying, salting out, or evaporation. Alkali insoluble bases remain in the iron sludge and must be separated from it by extraction with a suitable organic solvent. Frequently the reduction mixture, after removal of the iron, can be used directly in the next step without isolation of the reduction product.
The method described can be used also for the reduction of nitroso and azo compounds, and other substances, to amines.
The reduction of nitro compounds by iron can also be carried out in the presence of enough acid to cause all of the iron to go into solution. This procedure is used in industry in those cases where a nitro compound has been prepared in
concentrated sulfuric acid solution and cannot be separated from the acid in any simple manner. However, this procedure is advisable only when the reduction product can be isolated directly from the acid reduction mixture without precipitating the iron (see the preparation of H acid, page 211). In contrast to the first method, the high-acid reduction can be carried out very well using wrought iron scrap.
 |
Finally, iron can also exert a reducing action when used in the presence of caustic alkali; and nitrobenzene is converted successively to azoxy-, azo-, and hydrazobenzene under these conditions. Especially finely ground cast iron powder must be used for this purpose, ind it must be etched before its use in some cases.
Iron is not a suitable reducing agent for use when only one of several nitro groups present is to be reduced, or when a nitro compound is to be reduced without altering azo groups which are present. These “partial reductions” are usually carried out technically with hydrogen sulfide in the form of sodium sulfide (ЫагБ) or sodium hydrosulfide (NaSH). This reduction method is not limited to partial reductions; it frequently finds use in the anthraquinone series for reducing
nitro compounds which contain no other reducible groups. It may be carried out not only in aqueous solutions but also in alcoholic solutions and is very useful, therefore, in die reduction of substances which are difficult to handle because of complete insolubility in aqueous media. For further details, see m-nitroaniline (page 113).
Many of the reducing agents used in laboratory work find some application in dye manufacture. The most prominent of these are zinc and zinc dust, which are usable in various reductions in acid and alkaline solution, and in hot or cold solutions. Because of their high price, however, these reducing agents are used technically only in those cases where the cheaper reagents, such as iron or sodium sulfide, either fail to work or give less satisfactory results (cf. benzidine, page 124). This applies in even greater measure to the still more expensive reagents, tin and stannous chloride, which are used industrially only in exceptional cases.
Sodium hydrosulfite (ЫазБгО.#) is also a very good reducing agent (see page 157), but its general technical use is also excluded on the basis of cost. Nevertheless, hydrosulfite is a valuable reducing agent for special purposes, especially in the preparation and application of vat dyes (cf. page 321). Mention may also be made of the use of hydrosulfites and sulfoxylates in the destruction of dyes in discharge printing, in stripping of dyed textiles, and in bleaching.
Sulfurous acid and its salts are inexpensive reducing agents which are, however, usable only in special cases. These reagents frequently give sulfonation simultaneously with reduction (cf. the preparation of l-naphthylamine-2,4-disulfonic acid from 1-nitronaphthalene, and of l-amino 2 naphthol-4-sulfonic acid from nitroso-/3-naphthol, pages 178 and 201). Also, in the reduction of diazobenzene to phenylhydrazine, a N-sulfonic acid is formed first and this must be split by vigorous treatment with hydrochloric acid (see pages 96 and 128).
Reduction by glucose and alkali is used only in a very special field: the reduction of nitro compounds to azoxy and azo compounds.
It should also be mentioned that electrolytic reduction and reduction with molecular hydrogen in the presence of suitable catalysts are also used in the dye industry in isolated instances.
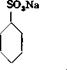 SO. H so.—
SO. H so.—
I I 2
/N HtSO« CaCOj NatCOs
+ S°r [or сТ(ОІадГ ( or Na, SoJ
In an iron, porcelain, or enameled vessel provided with stirrer and reflux condenser, 200 grams of benzene is mixed carefully over a period of about 30 minutes with 450 grams of oleum containing 10 per cent S03. The temperature should not be allowed to rise above 75° until the mixing is complete, then it is raised to 110°. Higher temperatures are not recommended, since the formation of disulfonic acid occurs readily. (Benzenedisulfonic acid is formed more easily than the literature would indicate. See the preparation of benzene-m-disulfonic acid, page 143.) After about 1.5 to 2 hours, the benzene has all disappeared.
The sulfonation mixture is poured into 1000 cc. water and neutralized, while boiling and stirring vigorously, with about 450 grams of pulverized chalk or the equivalent amount of slaked lime.
For laboratory purposes, the use of calcium carbonate is preferred because the COj which is generated gives good mixing and an excess of the insoluble carbonate does no harm. If the container is sufficiently large and the operation is conducted carefully, foaming-over can be avoided without difficulty. Lime, on the other hand, tends to form hard lumps which are only slowly broken up by the usual laboratory stirrers, so that it is easy to add too much and a subsequent neutralization of the excess is required.
The opposite situation prevails in industrial operations. Here, strong foaming is much more troublesome than in the laboratory, and breaking up lumps of lime offers no difficulty with the much more efficient large scale stirring apparatus. Hence in plant operations, the bulk of the acid is neutralized with slaked lime, and, in those cases where an alkaline solution must be avoided, only the last part is neutralized with chalk.
The neutral solution of the calcium salt is filtered through a large suction funnel (Fig. 13) to remove the calcium sulfate, and the latter
|
Fig. 13. Laboratory suction filter. |
is washed thoroughly with water. The combined filtrate and washings total about 1500 cc. The calcium salt is now converted into the sodium salt by the addition of enough sodium carbonate to make the solution just alkaline to phenolphthalein (about 110 grams of soda ash). (Sodium sulfate can be used instead of sodium carbonate.) The material is satisfactory for use in the preparation of phenol if about 99 per cent of the sulfonate is present as the sodium salt. The hot solution of the sodium salt! s filtered to remove the precipitated CaC03, and the clear filtrate is evaporated over a free flame until crystals of sodium benzenesulfon — ate begin to separate. On cooling this concentrate, a paste is formed, consisting of about 50 per cent solid material. The solid material consists of 90 per cent benzenesulfonate, 7 per cent sodium sulfate and carbonate, and a small amount of calcium salts. The paste can be used directly in the preparation of phenol by alkali fusion (see next preparation).
If the sodium benzenesulfonate is to be isolated, the mixture is evaporated to dryness on a water bath. If it is desired to have the product as free as possible from inorganic salts, the paste may be filtered and the precipitate dried. This procedure entails considerable loss, of course.
Technical Observations. In large scale production, quantities of benzene up to 1200 kilograms are sulfonated at one time. The gypsum is usually separated from the liquid by means of rotary filters similar to those used in soda manufacture. The specific gravity of the sodium salt solution is about 8-10° Be, and after concentration, about 25° Be (temperature 100°C.).
Benzenesulfonic acid can also be obtained by an entirely different process. Instead of sulfonating in the liquid state, benzene vapor can be passed through sulfuric acid of 66° Be at 100-140°. The water formed is continuously distilled out along with some unchanged benzene, so that at the end of the reaction there is obtained a solution of benzenesulfonic acid containing very little sulfuric acid. This solution can be neutralized directly with soda, without liming, and the product fused with caustic soda. Toluene can also be sulfonated smoothly by this method. This new process was discovered by the Bakelite Corporation.
 18 сентября, 2015
18 сентября, 2015  Pokraskin
Pokraskin 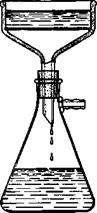
 Опубликовано в рубрике
Опубликовано в рубрике 