This is the simplest member of the pyrazolone dyes which are prepared in two ways: (a) from dihydroxytartaric acid and phenylhy — drazine (tartrazines), and (b) from phenylmethylpyrazolones by coupling with diazo compounds. The second method is simpler and has largely displaced the older, first method. The pyrazolone is prepared from a given phenylhydrazine (e. g., phenylhydrazinesulfonic acid, page 128) and acetoacetic ester, and coupled with diazotized aniline:
CH C — N2—
CHj-Cr^Sc-OH -* сн,-с/%с-он
N t!— 1N—^ S03H N U 1N—SO, H
l-Sulfophenyl-3-methyl-5-pyrazolone Fast light yellow G
In the formula for the sulfophenylmethylpyrazolone, the starred hydrogen is the one which is replaced by the azo group. This hydrogen is adjacent to a hydroxyl group (+) which makes the coupling reaction possible. The hydroxyl group here is quite similar to those in phenols and naphthols, and can contribute to lake formation in azo dyes derived from o-aminophenols or o-aminonaphthols. These dyes are of the type:
 |
For example, a very stable chrome wool dye, eriochrome red В (Geigy) is formed from l-amino-2-naphthol-4-sulfonic acid (page 201) and phenylmethylpyrazolone.
Eriochrome red В
Sulfophenylmethylpyrazolone, equivalent to 26 grams (0.1 mole) of 100 per cent material, is dissolved in 120 cc. water containing 6 grams of soda ash, and 30 grams of sodium acetate is added to the solution. The solution is cooled to 0°C. and mixed with a phenyldiazonium solution prepared from 9.3 grams of aniline. The mixture is stirred until a
test portion, after being salted out, no longer gives a red color with alkaline resorcinol solution. The coupling reaction takes about 4 to 6 hours. The mixture is then heated to boiling and salted out with 100 grams of salt. The yield is about 40 grams of strong dye.
These pyrazolone dyes, especially the more complicated ones, are much more stable than fast yellow (page 271). The dyes derived from o-sulfoamines, such as p-toluidine-m-sulfonic acid or p-chloroaniline-o-sulfonic acid, are among the most stable yellow dyes known. Fastness to light can be increased still more, however, by using chlorinated phenylpyrazolones, instead of the sulfophenyl compound, in preparing the dye. The xylene yellow dyes (Sandoz) are chloro derivatives of this type and are used rather widely, despite their relatively high cost, because of their unparalleled fastness to light. Tne possible structural variations are almost unlimited, and only one example will be given: Polar yellow 5G (Geigy). This dye has the following structure:
CH,
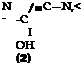 |
||||
N=C
being made up of (2) m-sulfo-p-chloroaniline, (2) acetoacetic ester, (3) p-amino — phenol, and (.4) p-toluenesulfonic acid.
Polar yellow 5G is prepared by condensing p-chloro-m-sulfophenylhydrazine with acetoacetic ester, and coupling the resulting pyrazolone with diazotized p — aminophenol in acetic acid solution. The alkali-sensitive dye which is formed is treated at 70°C. with p-toluenesulfonyl chloride, in the presence of soda and 1 mole of sodium hydroxide, to esterify the hydroxyl group. This esterification makes the dye insensitive to alkali and, at the same time, fast to milling on wool.
Technical Observations. The manufacture of pyrazolone dyes is relatively simple. The arylhydrazine condensations are usually run in enameled vessels to minimize losses of expensive materials. Nothing particularly new is involved in the diazotization and coupling reactions.
Hansa Yellow G
3- Nitro-4-toluidine (15.2 grams) is diazotized by the procedure given on page 246. Ice is added to the filtered diazo solution to lower the temperature to 2-5°C. The solution is then neutralized with sodium acetate to the point where Congo red paper is just turned violet.
Another solution is made by dissolving 17.7 grams of pure aceto — acetanilide in 300 cc. water (at 50°) containing the minimum amount of caustic soda necessary to form a clear solution. About 12 cc. 35 per cent caustic is required. This solution is cooled, and 25 grams of crystalline sodium acetate is added. Dilute acetic acid is then added carefully until a faintly acid reaction to litmus is produced but no precipitation occurs. The clear diazo solution is now added dropwise with very good stirring, and stirring is continued for 12 hours. The dye, which is then completely precipitated, is filtered off and washed thoroughly, the final time with hot distilled water. The product is ground in a mortar to a uniform paste, preferably with the substrate if an opaque color is being prepared. A weighed portion of the material is dried to determine the yield. The yield is almost quantitative.
Technical Observations. Hansa yellow G is a pure yellow. More greenish brands are obtained by using o-nitroaniline or o-nitro-p-chloroaniline in place of nitrotoluidine, and also by substituting acetoacet-o — or p-chloroanilide for the aceto- acetanilide. All of these dyes are extraordinarily fast to light, and they fulfill all the requirements of the pigment industry with respect to oil and spirit stability. They are among the most important pigment colors.87
|
Permanent Red 2G88 OH
|
The procedure given on page 247 is used to diazotize 18.3 grams (0.1 mole) of pure 2,4-dinitroaniline, and the resulting concentrated sulfuric acid solution of the diazo compound (not diluted with ice) is added slowly, with stirring and ice cooling, to a solution of 15.5 grams of /З-naphthol in 400 cc. alcohol. The addition is made at such a rate that the temperature does not rise above 5°C. Coupling begins immediately and is complete in a few minutes after all of the diazo compound has been added. Stirring is continued for 1 hour, and then the dye, which has separated completely, is filtered off with suction and washed with alcohol until drops of the filtrate appear colorless (about three washings). The dye is then washed with hot water until the washings are
8T M. L. B., Ger. Pat. 257.488 (1913) [Frdl, 11, 452 (1912-1914); C. A., 7, 2690 (1913)1; and Ger. Pat. Applic. F 33,190 (1913) [Frdl., 11, 455 (1912- 1914)].
88Lauch (Akt.-Ges. Апіііп-Fab. Berlin), Ger. Pat. 217,266 (1909) [Frdl., 9, 418 (1908-1910); C. A., 4, 1242 (1910)1.
neutral, dried in a steam heated oven, and powdered. The yield is 32 to 33 grams (95 to 98 per cent of the theoretical amount) of shiny, orange-red powder which is almost completely insoluble in water and only slightly soluble in the common organic solvents.
Technical Observations. Permanent red 2G (lithol fast orange R) is, like Hansa yellow, a valuable pigment color having outstanding fastness to light and very good oil and spirit fastness.
For laboratory preparations, the procedure described above — coupling in alcohol solution — is the simplest and surest method for obtaining a pure dye whose color strength corresponds to present strict specifications. The use of an excess of naphthol greatly accelerates the coupling reaction and thus prevents decomposition of the diazo compound.
Large scale preparations are usually carried out with an aqueous emulsion of naphthol prepared by precipitating the naphthol from a caustic alkali solution containing a dispersing agent (e. g., a sodium isopropylnaphthalenesulfonate) by slow addition of hydrochloric or sulfuric acid. In any case, the coupling reaction must be carried out in strongly acid solution, or dull brownish dyes are obtained which are entirely unusable.
Helio Bordeaux BL[55] OH
![]()
![]() >—N=H—j
>—N=H—j
 25 ноября, 2015
25 ноября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 