To prepare indigo from the sulfuric acid solution of isatinanilide, it is not necessary to isolate the pure anilide or its hydrochloride. A solution of sodium hydrosulfide is prepared by introducing hydrogen sulfide into a solution of 45 grams of sodium hydroxide in 150 cc. water. This solution is mixed with the sulfuric acid solution of a-isatinanilide obtained from 200 grams of “thioamide” by pouring the two solutions into 6 liters ice water. A definite, but slight, excess of hydrogen sulfide should be present at all times. The reduction requires about 30 minutes during which the thioisatin separates as a voluminous, brown precipitate. Aniline sulfate remains in the solution. The thiosatin is filtered off when a filtered test sample of the reaction mixture gives no further precipitate with more sodium sulfide, usually after about 1 hour. The precipitate is washed until the washings have a specific gravity of only 1.007 (1° Be) and is then stirred into 3 liters water. A concentrated solution of soda is added until a permanent, strongly alkaline reaction is obtained. This requires about 30 grams of soda. The formation of indigo takes place very rapidly, but it is desirable to heat the mixture at 60°C. for 1 hour and then allow it to stand overnight. The indigo and sulfur mixture is filtered off, washed thoroughly, and dried at 80°. The dry material is extracted with twice its weight of carbon bisulfide, leaving about 80 grams of pure indigo.
Technical Observations: The reactions involved in the Sandmeyer synthesis of
indigo are surprisingly smooth. The over-all yield of dye, calculated on the basis of aniline, is about 80 per cent. The process was used for a short time by Geigy, the cost of the dye being about 10.8 francs per kilogram of 100 per cent product. This product reduced to a vat more satisfactorily than other commercial indigos and was favored by dyers. The whole process was carried out without using alcohol, since all of the materials involved reacted well in aqueous solution when sufficiently finely divided. The chief difficulty was not with the hydrocyanic acid, but with the hydrogen sulfide. This compound is a dangerous industrial poison because its odor is not noticed after short exposure. The lead sulfide was treated with concentrated hydrochloric acid to form lead chloride and hydrogen sulfide which were returned to the process. The process of the Deutsche Gold — und Silberscheidean- stalt replaced the Sandmeyer process soon after large scale manufacture of indigo was started. The yields in the new process were as high as 85 per cent, so the older process could not compete with it.
// Ал
![]()
 |
|
 |
|
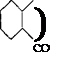
Thioindigo
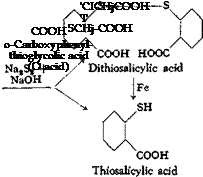
(a) o-Carboxyphenylthioglycolic Acid (0 Acid)
Anthranilic acid is diazotized and the diazonium salt is converted to a mixture of thiosalicylic acid and dithiosalicylic acid by the action of sodium polysulfide. For these reactions, the following three solutions are prepared:
I. 68.5 grams anthranilic acid (0.5 mole)
500 cc. water
78.5 grams hydrochloric acid (21° Be)
II. 34.5 grams sodium nitrite (100 per cent)
75 cc. water
III. 85 grams sodium sulfide, cryst. (NagS * 9НгО)
11.1 grams flowers of sulfur
100 cc. water
Solution I is prepared by adding the anthranilic acid-to the aqueous solution of the acid. Solution III is made by boiling the sulfur in the
sulfide solution until dear, and then adding J.2 grams of 40° Be sodium hydroxide solution to it. Solution I is cooled to 0°C. with vigorous stirring in a 1-liter beaker, and Solution II is added slowly to it. The temperature rises to about 5°, and starch-iodide paper gives a weak blue reaction. Solution III is now mixed with 500 grams of ice in a 4-liter beaker and, with vigorous stirring, the diazonium solution is added portionwise in the course of 10 minutes. The mixture foams heavily and a yellow precipitate is formed. The mixture is stirred for 2 hours and then made acid to Congo red by adding 90 grams of 21° Be hydrochloric acid. The precipitated thio — and dithiosalicylic acids are filtered off and washed until free from acid. Before the precipitate is worked up, the dithiosalicylic acid must be reduced to thiosalicylic acid. The mixture of acids is placed in a 2-liter beaker with 500 cc. water, 25.8 grams of soda ash is added gradually with stirring, and the solution, which must be acid to litmus, is heated to boiling. Reduction is effected with 100 grams of ВёсЬатр iron (page 77) at 95° for 2 hours, replacing, from time to time, the water lost by evaporation. To determine when reduction is complete, a sample is removed, made strongly alkaline, boiled for a short time, and filtered. The filtrate is then treated with hydrochloric acid, and the precipitated acid is filtered off, washed, and partially dried on a clay plate. It must be easily soluble in cold alcohol. (Dithiosalicylic acid is very difficultly soluble in alcohol.)
When the test is satisfactory, the reaction mixture is made alkaline by the addition of 60 grams of 40° Вё sodium hydroxide solution, and at 95°C. a solution of 52 grams of chloroacetic acid and 29 grams of soda ash in 150 cc. water is added. The mixture, which must be alkaline at all times, is held at 90° for 30 minutes, and is then cooled and allowed to settle. The residue of iron is filtered off and washed with dilute sodium hydroxide solution. The О acid is precipitated from the filtrate by adding 150 grams of 21° Вё hydrochloric acid. This precipitation is most satisfactory when the solution is cold, and it is desirable to carry it out as slowly as possible and with good mechanical stirring. The product is filtered off, washed until free from acid, and dried at 80°. The yield is about 85 grams, or 80 per cent of the theoretical amount.
 21 декабря, 2015
21 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 