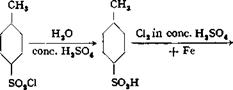
Hydrolysis of 2,6-dichlorobenzal chloride is much more difficult than that of benzal chloride itself. In contrast to the latter, 2,6-dichloro — benzal chloride is not hydrolyzed either by water and iron or by calcium or potassium hydroxide, even under pressure at 150°C. The hydrolysis to the desired aldehyde is effected with concentrated sulfuric acid, although a considerable portion of the product is resinified in the process.
A mixture of 100 grams of 2,6-dichlorobenzal chloride and 200 grams of 66° Вё sulfuric acid is stirred for 12 hours at 55°. The mixture is then diluted with 1 liter of water, and the product is separated from the diliite
sulfuric acid and distilled with steam. About 30 grams of pure 2,6-di- chlorobenzaldehyde, melting at 71°, is obtained.
Technical Observations. 2,6-Dichlorobenzaldehyde has become a rather important intermediate in recent years, as it is the starting material in the preparation of several dyes of the aurine series (eriochrome azurol, etc.). It is of technical interest also because its preparation involves three types of chlorination reactions. The first two of these offer little difficulty even in large scale preparations, but the third is not easy to accomplish. The latter suffers from the inadequacy of large glass apparatus; containers of copper, tin, etc., cannot be used and enamel cracks at the high temperatures which are necessary. Pyrex or porcelain containers are used, therefore, heated with superheated steam or by means of sand baths. Gas heating can also be used. When glass containers are used, steam heating affords protection from fires, but the pressure is so high in the steam heated pipes (200 atmospheres) that bursting may occur. For these reasons, the simple sand bath is still used to a large extent.
Traces of iron (from factory dust) or of antimony (from rubber stoppers or tubing) may prevent chlorination in the side chain.
 |
|
The preparation of 2,6-dichlorotoluene is one of the few examples of the technical use of tne Sandmeyer reaction. This method was once used in the preparation of o-chlorotoluene (for subsequent conversion to o-chlorobenzaldehyde) from o — toluidine. Another process[35] for preparing o-chlorotoluene makes use of the following reactions:
The first step involves hydrolysis, with concentrated sulfuric acid, of p-toluene — sulfonyl chloride, which is quite cheap. The resulting sulfonic acid is chlorinated smoothly in the position ortho to the —CHs group, the reaction being carried out in sulfuric acid solution using iron as a catalyst. Finally, the sulfo group is split out with steam, yielding the desired o-chlorotoluene in excellent yield. This process is less satisfactory for use in the laboratory, but gives the best results in industrial operations.
The chlorine atom in o-chlorobenzaldehyde is easily replaceable by a sulfo group by heating the chloro compound with neutral sulfite at 150°C. Ortho sulfo — nated benzaldehyde give alkali fast triphenylmethane dyes (patent blue, erioglau — cine, xylene blue).
In large scale operations, distillation at the intermediate stages is omitted, except that the dichlorotoluene must be distilled to make it completely dry. With the other compounds, it suffices to separate them from the mother liquors in leaded separatory funnels. The copper solutions are always reconverted to cuprous chloride by treatment with zinc dust or iron, and the loss involved in this recovery seldom exceeds 2 per cent.
2,6-Chloronitrotoluene and 2,6-chlorotoluidine are technically important in other respects also. The former compound is the starting material for the prepara — oitn of 4,4′-dichloroindigo, which yields, on further chlorination or bromination, the very greenish 4,5,4′,5′-tetrahalogenindigos (e. g., brilliant indigo 4G). 2,6-Chloro — toluidine, as fast scarlet TR base, is used in generating ice colors (naphthol AS). The isomeric 4-chloro-2-toluidine (fast red KB base), prepared by reduction of the by-product 4-chloro-2-nitrotoluene, is used for the same purpose.
![]()
![]()
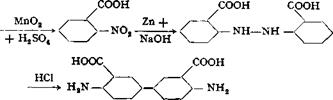 CH,
CH,
A solution is made of 33.4 grams (0.2 mole) of pure o-nitrobenzoic acid53 in 120 cc. sodium hydroxide solution (40° Вё) and 40 cc. water, by heating to 100°C. Zinc dust is added to this solution, in small portions with vigorous stirring, at such a rate that the temperature of the solution stays at 100-105° without external heating, continuing the addition until the originally deep yellowish brown solution is completely decolorized. About 40-50 grams of zinc dust is required, depending on its quality, and the addition requires about 15 minutes. As soon as the solution becomes colorless, 600 cc. hot water is added, and the excess zinc dust is filtered off and washed with hot water. The filtrate is treated with 20 cc. 2 N acetic acid and a few drops of bisulfite solution (to prevent oxidation), and then 140 cc. 21° Вё hydrochloric acid is added dropwise with good stirring. At this point, the solution should be strongly acid to litmus, but not to Congo red, so that rearrangement does not occur. (The acetic acid added earlier facilitates attainment of the correct acidity.) A filtered test portion of the solution should give no precipitate on addition of acetic acid, and additional acid is added if necessary. The solution is then cooled and the hydrazobenzoic acid, which separates in a crystalline, easily filterable form, is filtered off with suction and washed thoroughly with cold water. The pale brownish yellow filter cake, weighing about 50 grams, is stirred with 80 cc. water to form a uniform paste; 60 cc. 21° Вё hydrochloric acid is added, and
the mixture is heated at 95-100° for 30 minutes, maintaining the volume by addition of water. When the material has all dissolved except for a small black residue, the resulting solution is filtered hot and the residue washed with a hot mixture of 15 cc. concentrated hydrochloric acid and 30 cc. water. The violet colored filtrate is again heated to 70-80° and neutralized, with stirring, with ammonia (50 to 60 cc. 25 per cent solution) to the point where the acid reaction to Congo red disappears. The precipitation is then completed by the addition of a solution of 30 grams of crystalline sodium acetate in 70 cc. water. A filtered test portion should give no further precipitate with either acetic acid or sodium acetate. The precipitated benzidinedicarboxylic acid is filtered from the warm solution, washed thoroughly with warm water, and dried in a steam heated drying oven. The yield is about 26 grams of pale greenish gray product, or about 92 per cent of the theoretical amount.
An especially pure, pale yellowish green product can be obtained by cooling the acidic filtrate following the rearrangement reaction. The bulk of the material separates as the hydrochloride. This is filtered off and stirred with hot water, and converted to the free benzidinedicarboxylic acid by the addition of sodium acetate. The mother liquor remaining after filtering off the hydrochloride is treated according to the above procedure for recovering the rest of the product.
Technical Observations. Tetrazotized benzidinedicarboxylic acid gives, with 2 moles of l,8-aminonaphthol-2,4-disulfonic acid (Chicago acid, see page 217), a disazo dye which, in the form of its copper compound on viscose silk, gives a bright blue shade having extraordinary light fastness.
2.Nitro-4.aminotoluene from p-Toluidine
|
CH, CHS
NHj NH2 |
p-Toluidine (53.5 grams, 0.5 mole) is added with stirring to 1000 grams of concentrated sulfuric acid (66° Be), at such a rate that the temperature rises to about 40°C. but not appreciably higher. Rapid solution occurs under these conditions. The solution is cooled in an ice-salt bath (see page 131) to —5°, and a mixture of 34 grams of 93 per cent nitric acid, free from, nitric oxide, and 66 grams of concentrated sulfuric acid (66° Вё) is added slowly, keeping the temperature below 0°. If nitric acid or mixed acid free from nitric oxide is not available, it can be prepared by passing a stream of dry air through concentrated nitric acid at 40-50° until the acid is completely colorless. The acid is then cooled while air is being passed through, and a test sample is titrated with sodium hydroxide to determine its acid strength. The amount of the acid containing 31.5 grams of HN03 is weighed out and to it is added, slowly and with cooling, exactly twice its weight of concentrated sulfuric acid. Care must also be taken, of course, that the sulfuric acid used in dissolving the toluidine, as well as in preparing the mixed acid, is free from S02, because otherwise nitrous acid or nitrosylsulfuric acid will be formed in the reaction mixture.
After the addition of the nitrating acid, the reaction mixture is stirred at 0° for 5 to 6 hours and then tested (nitrometer) to establish that all of the nitric acid has been used. If the test is satisfactory, the mixture is poured, with stirring, onto 2.5 kilograms of ice. The difficultly soluble sulfate of 2-nitro-4-aminotoluene separates as reddish crystals which are filtered off with suction after a short time, pressed out well, and washed with cold water to remove adhering acid. The precipitate is then dissolved in boiling water, and the solution is filtered to remove a small amount of tarry residue, then made alkaline, while still warm, with ammonia. The mixture is thoroughly cooled, and the orange yellow crystalline base is filtered off, washed with cold water, and dried at room temperature. The yield is 60 to 62 grams, or about 80 per cent of the theoretical amount. The product melts at 76-77°.
An additional amount of the product can be obtained by neutralizing the sulfuric acid mother liquor with ammonia. It is recommended that the mother liquor be neutralized just to a point where it is still faintly acid to Congo red. On cooling the hot solution, a few grams of nitroaininotoluene sulfate separates, mixed with some tar. This is filtered off and converted to the free base. The filtrate is then made alkaline by the addition of more ammonia, precipitating several grams of the free base. These second and third fractions melt only about one degree lower than the main fraction. Together, they total about 7 to 8 grams, or 10 per cent, so that the total yield is about 90 per cent of the theoretical amount.
General, Remarks. Primary aromatic amines, whose basic properties are not too strongly reduced by the presence of negative substituents, can often be nitrated smoothly without protecting the amino group by acetylation or the like. The nitration is carried out in the presence of a large amount (at least 10 parts) of concentrated sulfuric acid and at as low a temperature as possible, below 0° in any case. The nitric acid must be entirely free from HN02.
Under these conditions, the orienting influence of the amino group is almost completely masked and the other substituents which are present determine the position taken by the nitro group. Thus, in compounds in which the position para to the amino group is occupied by a halogen atom, or by an alkyl, aryl, or alkoxy group, the nitro group almost exclusively enters the position ortho to these substituents and meta to the amino group. This is the case, for example, with p-toluidine,
panisidine, p-phenetidine, and p-chloroaniline, as well as with diamines such as benzidine, 4,4’_diaminodiphenylmetliane, etc. If the amine to be nitrated has one of the above mentioned substituents in the position ortho to the amino group, then the nitro group predominantly enters the position para to the amino group, and, to a smaller extent, the other free ortho position. For example, o-toluidine yields chiefly 4-nitro-2-aminotoluene along with a small amount of 6-nitro-2-aminotoluene; o — anisidine gives chiefly 4-nitro-2-aminoanisole, etc. These rules of orientation hold also for the nitration of secondary or tertiary amines in the presence of much concentrated sulfuric acid.
Only rarely is the sulfate of the nitrated base so insoluble that it separates directly on dilution of the reaction mixture as in the above example. In many cases, the hydrochloride can be precipitated by the addition of salt. Frequently, however, it is necessary to neutralize the entire amount of sulfuric acid in order to isolate the product, but this cannot be done with lime because then calcium sulfate would be precipitated with the base. In industrial preparations, the neutralization is done with magnesia, of which only a small amount is required because of its low equivalent weight, or with ammonia. In the latter case, the filtrate is treated with lime and the ammonia is recovered and used again. It is preferable to carry out the neutralization in steps, because most of the impurities are precipitated first, and the later fractions are pure.
Dinitrostilbenedisulfonic Acid and Diaminostilbenedisulfonic
Acid from p-Nitrotoluene (Simultaneous Oxidation
of Two Molecules)
(a) Dinitrostilbenedisulfonic Acid
100 grams, of p-nitrotoluene is sulfonated exactly like nitrobenzene (page 120), and the product is isolated as the sodium salt. The press cake is dissolved at 50°C. in a solution of soda ash in 500 cc. water. About 50 grams of soda ash is required, provided that the material has been pressed out adequately. The solution is filtered to remove some iron oxide which is almost always present, and diluted to 2 liters.
The solution is heated to 50° in a water bath, the apparatus being arranged to prevent local overheating by placing the reaction flask in the water bath on a cloth pad, thus permitting uniform heat transfer. Over a period of 30 minutes, 160 grams of 35 per cent sodium hydroxide is added to the stirred solution, during which none of the sodium sulfonate should precipitate. A mixture of about 1700 grams of 5 per cent sodium hypochlorite solution and 300 grams of 35 per cent sodium hydroxide is then added slowly over a З-hour period. The strength of the hypochlorite is first determined accurately by titration with AS2O3, and the quantity of solution corresponding to 85 grams of NaOCl is used. It must be remembered that hypochlorite solutions are stable only if they contain at least 5 per cent excess
NaOH; this fact is especially important in the preparation of hypochlorite solutions. The temperature should not be allowed to rise above 60° or yellow dyes of the Mikado series are formed. The mixture is allowed to stand at least 4 hours at 55°, during which time it should give a positive test for active chlorine with starch — iodide paper. The solution is then cooled to 15° and allowed to stand for a day after 400 grams of salt has been added. Sodium dinitrostilbenedisulfonate separates as a yellow, crystalline precipitate, and this is filtered off and washed with a small volume of salt solution.
The yield of the crude salt is about 100 grams.
 13 октября, 2015
13 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 