Arguably the most significant recent medical development which involves the use of dyes is photodynamic therapy (PDT). PDT is a treatment for cancer that uses a combination of laser light, a photosensitising compound (the dye), and molecular oxygen. In this treatment, the photo — sensitiser is given intravenously to the patient and some time is allowed (3-96 h) so that it equilibrates within the body. During this time the photosensitiser penetrates into the tumour cells. Irradiation of the cells with laser light may then initiate their destruction, thus providing a potential means for destroying the tumour. The method is rapidly gaining acceptance as a treatment for certain forms of cancer, which shows significantly fewer side-effects than conventional treatments. The photophysical mechanism of PDT is illustrated in Figure 10.13. Laser light interacts with the photosensitiser and promotes it from its ground state (1Sens) into its singlet excited state (1Sens*), which then undergoes intersystem crossing to a triplet excited state (3Sens*). This in turn interacts with molecular oxygen which, because its ground state is a triplet, returns the sensitiser to its ground state and in the process generates singlet oxygen (1O2). Singlet oxygen is the effective photodynamic agent which is highly reactive towards, for example, unsaturated centres in the proteins
|
Figure 10.13 Photophysical mechanisms involved in photodynamic therapy |
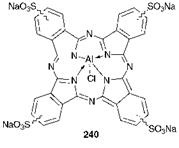
and lipids which construct the cell membrane. The most commonly used photosensitiser for PDT is referred to as Photofrin. To produce Photo — frin, haematoporphyrin, obtained from blood, is first treated with acetic and sulfuric acids followed by an alkaline work-up to give a derivative, HPD, which is a complex mixture of porphyrin monomers and oligomers. Photofrin is obtained from HPD by removal of the non-active monomeric components. There has been a vast amount of research carried out in the search for improved photosensitising agents, and the work is ongoing. Among the range of requirements for such an agent are that it should ideally absorb at > 600 nm for improved absorption of the laser light, improved specificity for tumour cells, and a triplet excited state which is sufficiently long lived to facilitate the formation of singlet oxygen. The dye should be soluble in body fluids and should clear from the body. A variety of modified porphyrins have been investigated for this purpose and particular promise has also been shown by a number of phthalocyanine derivatives, for example the sulfonated aluminium(iii) phthalocyanine 240.
|
|
 15 января, 2016
15 января, 2016  Pokraskin
Pokraskin 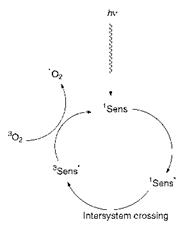
 Опубликовано в рубрике
Опубликовано в рубрике 