The structural chemistry of azo compounds is complicated by the possibilities of isomerism. There are two types of isomerism, which may commonly be encountered with certain azo compounds: geometrical isomerism and tautomerism.
Some simple azo compounds, because of restricted rotation about the (-N=N-) double bond, are capable of exhibiting geometrical isomerism. The geometrical isomerism of azobenzene, the simplest aromatic azo compound which may be considered as the parent system on which the structures of most azo colorants are based, is illustrated in Figure 3.1. The compound is only weakly coloured because it absorbs mainly in the UV region giving a lmax value of 320 nm in solution in ethanol, a feature which may be attributed to the absence of auxochromes (see Chapter 2).
|
Figure 3.1 The photo-induced geometrical isomerism of azobenzene |
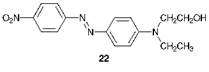
The compound exists normally as the trans or (E)-isomer 21a. This molecule is essentially planar both in the solid state and in solution, although in the gas phase there is evidence that it deviates from planarity. When irradiated with UV light, the (E)-isomer undergoes conversion substantially into the cis or (Z)-isomer 21b which may be isolated as a pure compound. In darkness, the (Z)-isomer reverts thermally to the (E)-isomer which is thermodynamically more stable because of reduced steric congestion. Some early disperse dyes, which were relatively simple azobenzene derivatives introduced commercially initially for application to cellulose acetate fibres, were found to be prone to photochromism (formerly referred to as phototropy), a reversible light-induced colour change. C. I. Disperse Red 1 (22) is an example of a dye which has been observed, under certain circumstances, to give rise to this phenomenon.
|
|
The phenomenon is explained by the colour changes associated with the E/Z isomerisation process, which may be initiated by exposure to light, the two geometrical isomers of the azo dyes having noticeably different colours. The undesirable effect is no longer encountered to any significant extent in the modern range of azo disperse dyes as the offending dyes were progressively replaced by more stable products. Curiously, there has been a relatively recent significant revival of interest in dyes which can exhibit controlled reversible photochromism for applications such as ophthalmic lenses, car sunroofs and optical data storage in which the colour change is utilised. Among the most promising photochromic dyes of this type are the spirooxazines (see Chapter 10 for further discussion).
Many commercial azo colorants contain a hydroxyl group ortho to the azo group. As illustrated in Figure 3.2, this gives rise to intramolecular hydrogen-bonding which further stabilises the (E)-isomer and effectively prevents its conversion into the (Z)-form.
|
Figure 3.2 Intramolecular hydrogen bonding in o-hydroxyazo compounds |
|
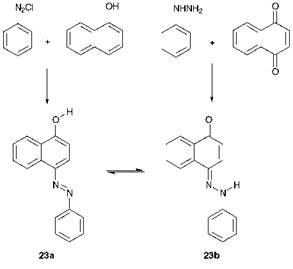
Scheme 3.1 Reaction scheme which provides evidence for tautomerism in some hydroxyazo dyes |
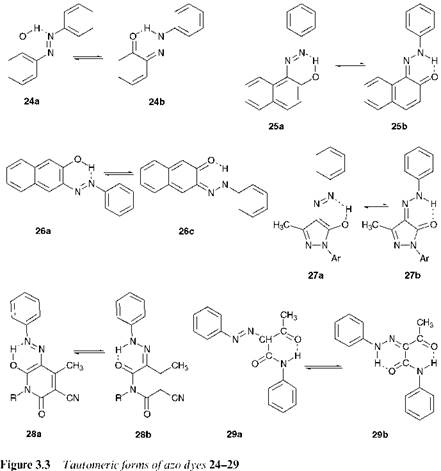
Another important feature of azo compounds in which there is a hydroxy group conjugated with (i. e. ortho or para to) the azo group, and very many commercial azo dyes and pigments show this structural feature, is that they can exhibit tautomerism. The classical experimental evidence for hydroxyazo/ketohydrazone tautomerism in dyes of this type was provided by Zincke. In 1884, he carried out the reactions of ben — zenediazonium chloride with 1-naphthol and of phenylhydrazine with naphtho-1,4-quinone. It might have been expected that the former reaction would give hydroxyazo compound 23a, and the latter would give the ketohydrazone tautomer 23b. In the event, it was found that both reactions gave the same product, a tautomeric mixture of 23a and 23b, as illustrated in Scheme 3.1. These tautomeric forms may nowadays be readily identified by their distinctive UV/visible, IR and! H, 13C and 15N NMR spectral characteristics in solution, and by X-ray crystallography in the solid state. In terms of colour, the ketohydrazone isomers are usually bathochromic compared with the hydroxyazo forms, and give higher molar extinction coefficients.
|
Figure 3.3 shows the tautomeric forms of a further range of hydroxyazo dyes. In solution, the tautomers exist in rapid equilibrium although commonly one or other of the tautomers is found to predominate to an extent that is dependent on their relative thermodynamic stability. In the case of 2-phenylazophenol, the azo isomer 24a predominates over the hydrazone isomer 24b. On the basis of a consideration solely of the summation of all of the theoretical bond energies in the molecule, it has been suggested that the hydrazone isomer would be expected to be more stable. However, this is outweighed by the considerably reduced resonance stabilisation energy in the hydrazone form, due to the loss of the aromatic character of one ring. In the case of hydroxyazonaphthalenes, 4-phenylazo-1-naphthol(23) and 1-phenylazo-2-naphthol(25), the reduc- |
tion in the resonance stabilisation energy in the ketohydrazone forms is due to the loss of aromaticity of only one of the naphthalene rings. In relative terms, the reduction in energy is less than in the benzene series, so that the two tautomers become much closer in stability. The position of the equilibrium, particularly in the case of compound 23, then becomes heavily dependent on the environment in which the dye finds itself, most importantly the nature of the solvent. Interestingly, 3-phenylazo-2-naph — thol (26) exists exclusively in the azo form 26a, because of the instability of hydrazone form 26b in which there is complete loss of aromatic character of the naphthalene system. In the case of azo dyes derived from heterocyclic coupling components, such as the azopyrazolones 27 and the azopyridones 28, and from ^-ketoacid-derived coupling components, such as the azoacetoacetanilides 29, the compounds exist exclusively in the hydrazone forms 27b, 28b and 29b respectively. X-ray crystallographic structure determinations have been carried out on a wide range of azo pigments, and these compounds have been shown, without exception, to exist exclusively in the ketohydrazone form in the solid state (for a more detailed discussion of this see Chapter 9).
In o-hydroxyazo compounds, but not in p-hydroxyazo compounds, there is strong intramolecular hydrogen bonding both in the hydroxyazo and ketohydrazone forms, as illustrated for compounds 24-29 (Figure 3.3). A factor which contributes to the explanation for the predominance of the hydrazone isomer in many cases is that in this form the intramolecular hydrogen-bonding is significantly stronger than in the azo form. This may provide an explanation for the observations that in the case of 1-phenylazo-2-naphthol (25), the hydrazone form 25b predominates while in the case of the isomeric 4-phenylazo-1-naphthol (23), in which there is no intramolecular hydrogen bonding, the two possible tautomers are of comparable stability. Intramolecular hydrogen bonding is a commonly encountered stabilising feature in a wide range of dyes and pigments of most chemical types, enhancing many useful technical properties. For example, lightfastness is usually improved. This is explained by a reduction in the electron density at the azo group due to hydrogen bonding, which decreases its sensitivity towards photochemical oxidation. Also, hydrogen bonding reduces the acidity of a hydroxyl group, giving the dye enhanced stability towards alkali treatments.
 11 сентября, 2015
11 сентября, 2015  Pokraskin
Pokraskin 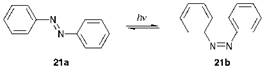
 Опубликовано в рубрике
Опубликовано в рубрике 