In general, tetrazolium salts are prepared by (a) oxidation of formazans and (b) alkylation of tetrazoles. Therefore, synthetic methods for formazans and tetrazoles are discussed first, followed by some direct and miscellaneous methods.
Synthetic methods for formazans have been reviewed.1,2
73.1.1. From Diazonium Salts
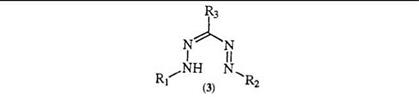
Electron-rich compounds such as hydrazones react with diazonium salts either at a nitrogen or at a carbon atom to yield formazans, either directly or through a subsequent rearrangement. The use of aldehyde
|
|
|
|
|
|
|
|
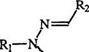
![]()
![]()

Scheme 1
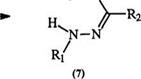
hydrazones is the standard procedure for the preparation of triaryl for — mazans. Diazonium salts couple to the amine nitrogen in the hydrazone (5) with displacement of a hydrogen to give the intermediate (6) which then rearranges to the formazan (7) (Scheme 1).4-7
 |
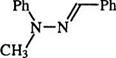 |
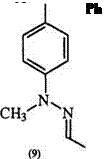
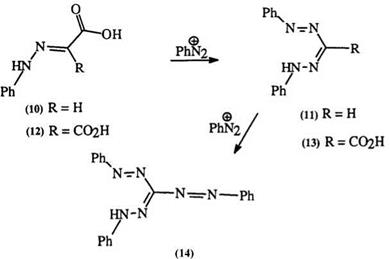
This mechanism is supported by the fact that a secondary hydrazone such as (8) yields the azohydrazone (9) rather than the formazan.8 Ketone hydrazones also yield azohydrazones. The coupling of hydrazones of glyoxylic acid (10) with diazonium salts is accompanied by decarboxylation to yield 3-unsubstituted formazans (11). Similarly, hydrazones of mesoxalic acid (12) yield formazans with a carboxyl group in position 3, e. g., 13 (Scheme 2).9,10 Both 11 and 13 can react with diazonium salts to yield the
![]()
|
|
|
|
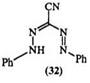
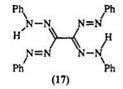
azo-substituted formazan (14).474 This method is suitable for the preparation of bisformazans linked through nitrogen, e. g., 15,12- 16 or through the 3-carbon atom, e. g., 16 including the directly linked formazan (17).13,17- 19 Table 1 illustrates the scope of this reaction.
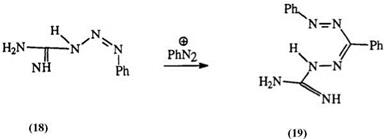
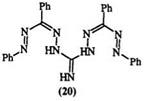
Guanyl hydrazones (18) react with diazonium salts to yield a special class of formazans called guanazyls, e. g., 19 (Eq. 1).20-22 The reaction is
|
|
|
|
Table 1. Synthesis of Formazans (3) from Hydrazones
|
|
|
Ri |
R2 |
R3 |
Yield (%) |
Refs. |
|
Ph |
Ph |
H |
19 |
30, 37 |
|
Ph |
Ph |
CH3 |
44 |
54 |
|
Ph |
Ph |
Ph 3 |
23 |
4, 19, 51,74, 102 |
|
Ph |
4-HO2 C-C6 H4 |
CH3 |
100 |
449 |
|
Ph |
4-H2NSO2-C6H4 |
n-C11 H23 |
100 |
101 |
|
2-H3 C-C6 H4 |
1-C1-C6H4 |
CH2 CH2 OH |
23 |
351 |
|
Ph |
Ph |
Glucose |
33 |
365 |
|
Ph |
a-C10 H7 |
Ph |
80 |
299,302,341 |
|
Ph |
4-(CH3 )3 N+-C6 H4 |
2-HO-C6H4 |
80 |
1 |
|
Ph |
4-(CH3 )3 N+-C6 H4 |
4-H3 CO2 C-C6 H4 |
48 |
1 |
|
Ph |
4-C6H4N=NC6H5 |
CH3 |
28 |
643 |
|
Ph |
4-C6H4N=NC6H5 |
Ph |
50 |
643 |
|
2-Pyridyl |
2-C1-C6 H4 |
2-Fury1 |
64 |
14 |
|
Ph |
Ph |
4-Pyridyl |
46 |
340 |
|
Ph |
Ph |
CO2 Et |
80 |
26, 30, 37 |
|
Ph |
Ph |
COCH3 |
70 |
26,334,366,367 |
|
Ph |
4-C6 H4 CH=CHPh |
4-Pyridyl |
39 |
340 |
|
Ph |
Ph |
1,4-Ethylene |
90 |
12 |
|
Ph |
4,4′-Biphenylene |
CH3 |
36 |
48 |
|
Ph |
4,4′-Biphenylene |
Ph |
76 |
15 |
|
Ph |
4,4′-Biphenylene |
2-Thienyl |
33 |
15 |
quite versatile with 3-aryl substituents and can lead to bisguanazyls, e. g., 20. However, 3-alkyl substituents limit the scope of the reaction.23-25
|
|
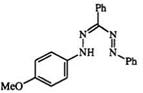
Synthesis of 1-(4-Methoxypheny1)-3,5-diphenylformazan (21).5 A solution of 12.8 g ofp-anisidine was dissolved in 50 ml of 6 M hydrochloric
|
(21) |
acid. The solution was cooled to -10°C and diazotized with a solution of 8.0g of sodium nitrite in 20ml of water. The diazonium salt solution was added in small portions (over 30min) to a stirred solution of 19.8 g of benzaldehyde phenylhydrazone in 110 ml of pyridine and 100 ml of ethanol at -10°C. The resulting solution (part suspension) was stirred for an additional hour at 0 °C, then poured into 3 liters of water. The precipitated formazan was filtered and air dried. The crude formazan was recrystallized from 500 ml of ethanol yielding 17.0 g (52%) of product mp 149-150 °C.
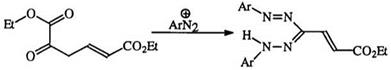
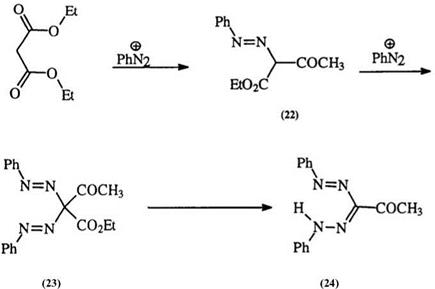
Diazonium salts add to active methylene compounds, for example ethyl acetoacetate, to form an intermediate azo compound (22), followed by the addition of a second diazonium salt (under more alkaline conditions) to yield the tetrazene (23) which then forms a 3-substituted formazan (24)10
|
|
through the loss of one of the electron-withdrawing substituent groups (usually an ester group) (Scheme 3). The isolation of the formazan (13) has also been reported.11,26 The relative displacement ability of a number of electron-withdrawing groups has been discussed.35 The intermediate hydra — zone (22) can be isolated under mild conditions and can be treated with a different diazonium salt to yield the unsymmetrical formazan (25).45 This method is very useful for the preparation of 3-substituted formazans,
![]()
![]()
 (2)
(2)
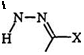
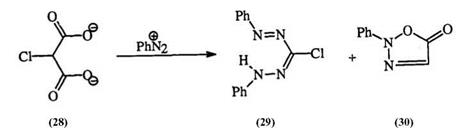
particularly when the starting aldehydes for hydrazone formation are not readily available. Unsaturated derivatives of reactive methylene compounds (26) can react with diaz°nium salts to form substituted formazans (27) (Eq. 2).44 The case of potassium chloromalonate (28) is interesting in that the chloro-substituted formazan (29) is obtained with traces of the
Table 2. Synthesis of Formazans from Methylene Compounds
R
 |
N=N
R
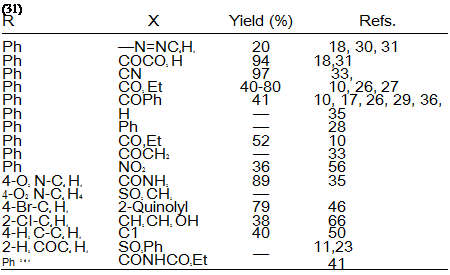
oxadiazolone (30), resulting from diazonium displacement of the halogen (Eq.3). Table 2 illustrates the scope of the utility of active methylene compounds in the syntheses of formazans.
Synthesis of 3-Cyano-1,5-diphenylformazan (32). 35 A solution of 18.8g of aniline in 100ml of 6M hydrochloric acid was diazotized at
— 10 °C with a solution of 16 g of sodium nitrite in 40 ml of water. To this solution was added a solution of 8.5 g of cyanoacetic acid in 100 ml of water cooled to 0 °C. The resulting solution was neutralized with 200 ml of a 20% solution of sodium hydroxide when an intense red color formed. The formazan was precipitated with 10% hydrochloric acid, filtered, and air dried. The crude formazan was recrystallized from 400 ml of ethanol to yield 9.5 g (40%) of golden platelets mp 158 °C.
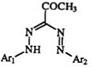
Aldehyde semicarbazones with two substituents on the terminal amino group (but not the hydrazino) (33) react with diazonium salts to yield N-carbamoyl-substituted formazans (34) (Eq. 4).47,48 A sulfonic acid deriva-
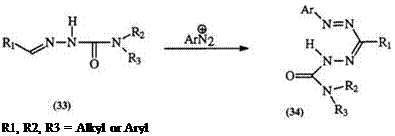 (4)
(4)
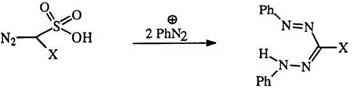
tive of ethyl diazoacetate (35) reacts with aryl diazonium salts in alkaline solution to yield an alkoxycarbonyl-substituted formazan (36) (Eq. 5).49-51 Diazomethane disulfonic acid behaves similarly.
|
X = CO2Et or SO3H
CH3′ (38)
 13 сентября, 2015
13 сентября, 2015  Malyar
Malyar 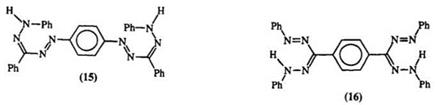
 Опубликовано в рубрике
Опубликовано в рубрике 