Around that time, there was a sudden realization that many different kinds of probe could give atomic resolution at surfaces. Electrical conduction, heat conduction, or acoustic transmission could all be used in principle. The scanning tunneling microscope, based on electron conduction, was immediately successful because it was so simple. A probe could be made by taking a metal wire and cutting it with scissors to give a pointed end. An atomically smooth surface could be prepared by pulling graphite flakes apart with sticky tape. Then a picture of the
|
Figure 3.17. Schematic diagram of atomic force microscope (AFM) |
carbon atoms on the surface could be obtained using the piezoelectric scanner. But electron conduction was only one way in which atoms could be detected. That could only be used on electrical conductors, thus preventing measurements on polymers, oxides, biological materials, etc.
Therefore, Binnig, Quate and Gerber14 devised the atomic force microscope (AFM) shown schematically in Fig. 3.17. The probe was now much smaller and lighter so as to detect the molecular adhesion forces. These forces caused a slight movement of the probe which could be observed by several different sensor methods. However, laser detection, as shown in Fig. 3.17, turned out to be most convenient. Small silicon cantilever probes were made by etching a silicon wafer. Laser light was reflected from the top of the silicon cantilever probe, and entered a detector where any deflection could be registered.
A typical adhesion experiment using the AFM was performed by raising the sample towards the probe while observing the cantilever deflection. When the probe was distant from the sample, nothing happened, but as the probe came within range of the adhesion forces, the probe was attracted and pulled down, as shown by the result of Fig. 3.18.
As the sample is pushed nearer to the probe, the attractive force increases rapidly and the probe then jumps into contact with the sample. This appears as a sudden deflection of the cantilever. Then, once the probe is stuck to the sample surface, the movement of sample and cantilever are equal, as shown by the linear contact deflection in Fig. 3.18.
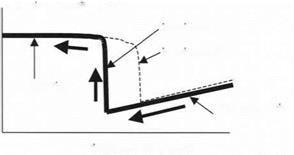
Moving the sample in the opposite direction can then be used to pull the probe off the surface. This result is shown in Fig. 3.19. The deflection of the cantilever is reversed along the same linear path as the probe sticks to the sample at first. Then, at a certain deflection of the cantilever, the probe jumps off the
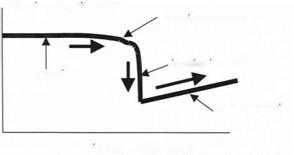
 deflection of cantilever probe
deflection of cantilever probe
adhesion force observed
jumping into contact
no attraction
contact deflection
Distance moved by sample
Figure 3.18. Result obtained from AFM adhesion experiment
surface. This separation of the probe usually occurs at a different point from the attractive jumping into contact. Thus, there is a hysteresis in the adhesion process; the making of contact is not reversible during the breaking stage.
It is evident from these results that two of the adhesion processes (making contact and breaking contact) can be observed in the atomic force microscope. The size of the contact spot can also be detected if some additional technique for sensing contact spot size is used, for example, electrical contact or thermal contact. Thus the three stages of adhesion can be found in the AFM. It has become clear from such measurements that all materials adhere in this test, verifying the first law of adhesion. These results will be considered in more detail in Chapter 4.
Another great benefit of the AFM is that the sample and probe can be immersed in liquid, so that adhesion can be tested in a wide range of environments. The immediate conclusion from such tests is that the adhesion is reduced by such immersion, thus verifying the second law of adhesion. Finally, the curious phenomena of time dependence, of irreversibility, and of hysteresis have
deflection of cantilever probe
jumping apart jumping together
no attraction
contact deflection
|
Distance moved by sample
|
been observed generally in these nanoscale experiments. Consequently, it is clear that the mechanism of adhesion is complex, as stated in the third law. It is now necessary in the following chapters to deal with these laws in greater depth.
 14 сентября, 2015
14 сентября, 2015  Pokraskin
Pokraskin 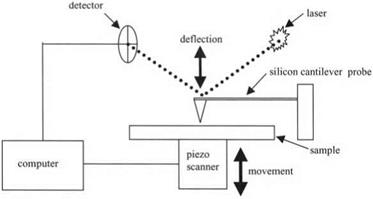
 Опубликовано в рубрике
Опубликовано в рубрике 