Electrochemical methods have been used extensively to elucidate the mechanism of reduction of tetrazolium salts. In aprotic media, the first step is a reversible one-electron reduction to the radical 154 as confirmed by ESR spectroscopy.256,266 As shown in Scheme 26, this radical can then disproportionate to the tetrazolium salt and the formazan anion (166) or take up another electron to the formazan dianion (167). The formation of the dianion through a direct reduction or through the intermediate tetrazolyl anion (168) has also been proposed.272 28 1,294 In aqueous solutions, where protonation/deprotonation equilibria contribute to the complexity of the reduction process, the reduction potentials are pH dependent and a one — electron wave is seldom observed.
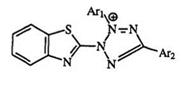 |
The reduction potentials, measured by scanning voltammetry, are substitution dependent.282 As shown in Table 12 for a series of triaryl tetrazoliums (169), the reduction becomes easier when electron-withdrawing substituents are present. This is in agreement with the polarographic data on a series of benzothiazolyl tetrazolium salts (170).283 With bis tetrazolium
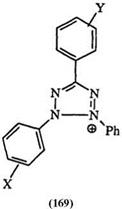 |
Table 12. Reduction Potentials
of Tetrazolium Salt (169)
X Y E (mV)a
‘Versus Ag/AgCI.
salts in nonaqueous aprotic solvents, a series of one-electron steps have been identified leading to both the “monoformazans” and the “diformazans” 164 and 165 in Scheme 25.285 In aqueous and micellar media, two two-electron steps and one four-electron step have also been observed.284 — 288
 29 сентября, 2015
29 сентября, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 