Obreimoff,6 working in the Physics Institute of Leningrad, where large sheets of a perfect type of Muscovite mica were available from the White Sea area near Chupa, knew of the problems of putting polished flat surfaces together, and recognized that optical contact could produce adhesion, but not satisfactorily. “Perfect” optical contact still contained gaps up to 100 atoms wide. However, he had observed that freshly split mica foils could be put back together to adhere with considerable force and set out to investigate this unique effect. His paper was most significant because it identified for the first time the three processes involved in adhesion; the jumping into contact, the equilibriation of the joint, and the pulling apart of the mica sheets. In addition, Obreimoff saw that evacuating the apparatus improved the adhesion, and also found electrical discharges which proved that adhesion was essentially an electromagnetic phenomenon.
The mica was cut using a razor blade into 2 x 20 x 50 mm blocks, and a glass wedge with a smooth and rounded end was used to split a foil 0.1 mm thick from the top surface. The experiment was placed in an evacuated tube and a glass hammer containing an iron mass was moved with a magnet to press the wedge into the mica to promote splitting (Fig 4.3). The region at which separation occurred was viewed under a microscope and Newton’s interference fringes were seen in the narrow gap between the split surfaces. By measuring the positions of
|
Figure 4.3. Apparatus used by Obreimoff to cleave mica and observe its subsequent adhesion. |
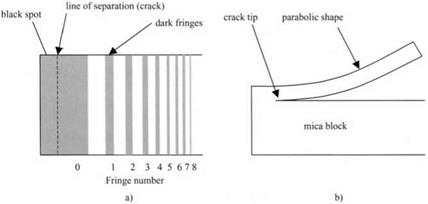
the interference fringes, Obreimoff was able to determine the shape of the mica strip which was being wedged from the block, as shown in Fig. 4.4.
The point of separation of the mica foil from the block, that is the crack line, could not be seen directly because this kind of interference experiment only detects gaps down to about 50 nm. But the shape of the bent mica foil could be accurately measured and was shown to be parabolic. In other words, the strip was behaving as a simple leaf spring and its shape was not affected by the molecular adhesion forces. This was an important observation because it proved that the molecular forces were only acting across the small gap near the line of separation. Thus the molecular forces could be neglected in terms of the large scale behavior of the system. Engineers therefore safely ignore such forces when calculating the shapes of machinery. The mathematics of these calculations is given in Chapter 14, essentially employing the energy conservation method of Obreimoff.
|
Figure 4.4. (a) Interference fringes seen in gap between mica foil and block, (b) Interpretation of shape of bent foil. |
By carrying out a series of splitting experiments on different mica samples, Obreimoff was able to show by balancing energies that, in air at ordinary pressure, the energy required to fracture the material was 1.5 Jm“2.
After splitting the mica, Obreimoff found that the surfaces would spontaneously jump back together when he removed the glass wedge. He measured the energy of this spontaneous adhesion and it was about 0.8 Jm"2, substantially less than the original adhesion energy. Then he pushed the wedge back in to measure the adhesion formed between the foil and the block by the jumping process and found the adhesion energy was around 1.2 J m~2, so it was taking more energy to split the adhering mica than was recovered on the jumping together. Thus he found some energy loss or adhesive hysteresis in this process. He also realized that it took some time for the splitting to reach equilibrium; the fringes moved for quite a time after the wedge was fixed, around 15 s. This was the first observation of the rate effect on adhesion.
Perhaps of most significance was the result obtained when the air was evacuated from the vessel around the mica. Adhesion was increased as the air was pumped out. This observation supports the second law of adhesion; that adhesion is reduced by air molecules which contaminate the mica surfaces (see Chapter 6). As these contaminant molecules were removed by evacuation, the energy of adhesion was then increased to 20 Jm 2, and impressive electrical discharges were seen around the mica samples at 1 nb pressure. This proved that the adhesion was connected with electromagnetic forces between the atoms in the mica crystal. However, it did not seem possible for the bonds themselves to have such a high energy, so the conclusion was that huge energy dissipation was occurring during this high vacuum adhesion test.
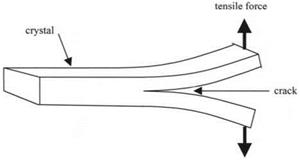
Further development of this cleavage technique was made by Gilman while he was at the General Electric Research Laboratory in Schenectady, New York. He attempted to measure the surface energy of a wide range of crystals by the Obreimoff method.7 He made two main changes to the experiment: first, he worked in liquid nitrogen to avoid any plasticity effects; second he found the wedge method to be too frictional and used direct tension applied to the split ends of the crystal to drive the crack. The rig is shown in Fig. 4.5.
Gilman concentrated on separation of the crystal planes by the crack; he did not make any observations of the crack healing to produce adhesive joining of the cleaved surfaces. So he did not fully demonstrate reversibility. However, he restricted plasticity to low levels and obtained surface energies comparable with those estimated from elastic modulus, as shown in Table 4.1.
The next improvement came from the strict control of the separation of the surfaces, to show the jumping into contact in the most dramatic way, as demonstrated by Tabor and Winterton.
|
|
|
|
|
Tabic 4.1
|
 15 сентября, 2015
15 сентября, 2015  Pokraskin
Pokraskin 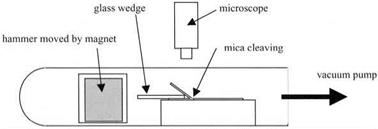
 Опубликовано в рубрике
Опубликовано в рубрике 