The problem of extrapolating down to molecular dimensions from macroscopic experience can be seen in two simple cases.6 First, consider the macroscopic task of sieving pebbles in the garden, as shown in Fig. 3.4(a).
If the mesh size is 20mm, then the 10mm pebbles can easily be shaken through the holes. Each pebble shows no adhesion to the mesh, nor to its neighboring pebbles. But imagine trying the same experiment with l00 nm diameter pigment particles. First of all, the pigment particles tend to stick together to form clumps, as shown in Fig. 3.4(b). Secondly, the small particles stick to the sieve and clog up the mesh, even when the holes are l000 nm, far larger than the particle size. Sieving such tiny particles is impossible unless the adhesion is reduced, say by adding surface contaminant molecules.
|
Figure 3.4. Sieving 10mm pebbles is straightforward if the mesh is 20mm. (b) Sieving 100nm pigment is impossible, even through a 1000nm mesh. |
|
Figure 3.5. (a) Key and lock mechanism cannot operate at the molecular level because (b) the key adheres to the lock prematurely. |
In the same way, consider the problem of getting a molecular size key into its receptor lock (Fig. 3.5(a)). The key sticks prematurely to the lock before it can enter, so the mechanism cannot work, as shown in Fig. 3.5(b), unless the molecular adhesion is reduced, by coating the receptor with contaminant atoms.
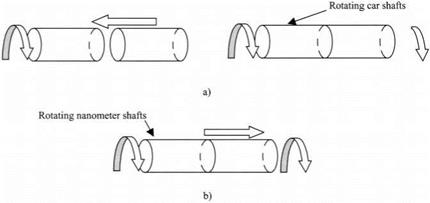
A second example of the difference between macroscopic and molecular behavior is seen in the designing of machines. We know how to design machines like cars. To make a clutch mechanism between two rotating shafts, we have to press the two shafts together to get them both to rotate synchronously, as illustrated in Fig. 3.6(a). Alternatively, we could glue the shafts together to make a solid joint between the two shafts.
The case of two rotating shafts in a molecular, nanoscale machine is quite the opposite. The shafts adhere spontaneously, leaping into contact, and provide
|
Figure 3.6. (a) Car shafts must be pushed together to give clutch mechanism, (b) Nanoscale shafts adhere spontaneously and must be pulled apart. |
|
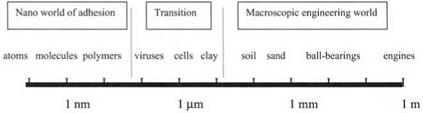
Figure 3.7. A new picture of the difficulty of extrapolating macroscopic adhesion mechanisms down to the molecular level. |
the clutch mechanism naturally. The problem here is getting the nanoscale shafts apart again. Either a force must be applied to separate the shafts or debonding molecules must be inserted at the junction to separate them.
These examples demonstrate the fallacy of extrapolating our macroscopic experiences down to the molecular level. The commonsense macromodels of suction, of adhesives, and of keying are not applicable at the nanoscale and lead us to error when interpreting molecular adhesion. It is useful to plot a logarithmic scale showing the boundary between the macro and nano worlds (Fig. 3.7). At the macroscopic engineering scale to the right of the diagram, above 10 pm. adhesion is not normal, and we need adhesives, keys and friction to get things to stick together. By contrast, to the left of the diagram, below l00 nm, adhesion is dominant and it is difficult to keep things apart. Everything is adhesive at this molecular scale unless we insert contaminant molecules to reduce adhesion. In between, from l00 nm to lii [i:n, we have the transition zone, where adhesion is variable. Sometimes things adhere and sometimes not, depending critically on the mechanism. It is interesting that many technological materials like latex, butter, cosmetics, paper, ink, cements, etc., together with living things like diatoms, viruses, bacteria, algae, and so forth, inhabit this transition zone. Here, adhesion is highly sensitive to chemical influences and to physical conditions. So sticking may be finely controlled, may vary enormously, and may also evolve over time.
Having defined the main adhesion fallacies, and determined where adhesion can always be found and predicted at the nanometer level, it is now possible to summarize the laws of adhesion which we believe to be universal.
 7 сентября, 2015
7 сентября, 2015  Pokraskin
Pokraskin 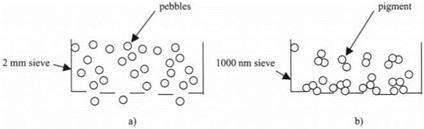

 Опубликовано в рубрике
Опубликовано в рубрике 