With regards to the adhesion mechanisms involved in textile reinforcement of rubbers, a distinction must be made between adhesion at the dip/rubber interface and adhesion at the dip/textile interface.
|
Figure 8.67 Possible condensation or addition reaction resols with latex double bonds. |
Adhesion at the dip/rubber interface is obtained by the direct crosslinking of dip components (double bonds, hydroxyl groups, etc.) with the rubber matrix, in particular with its sulfur components. This explains the fact that crosslinking agents have a direct effect on adhesive strength, and that systems containing low amounts of sulfur or sulfur-free systems can hardly reach high adhesion levels.
The resin system used is only partly responsible for adhesion, which is obtained either via reactions of active H-atoms or via the formation of chroman structures (Figure 8.67). This effect, however, has only limited importance due to the relatively low amount of resin contained in the system (ca. 20% related to the solids content) and the slow reaction rates. The formation ofan interpenetrating network is thought to be mainly responsible for adhesion.
Still fewer scientifically proven explanations are available for the dip/textile interface. Indeed, there is hardly any convincing and up-to-date argument in publicly available literature explaining the reasons for the underlying adhesion phenomenon. It is generally accepted that the adhesion is due partly to mechanical anchorage of the resin that penetrates into the spaces between the filaments, although microscopic investigations have shown that the dip only penetrates into two or three filament layers of the yarn (Figure 8.68).
This may be plausible with regard to the surface of rayon fibers that are microscopically quite rough, but it seems not to apply to ‘purely’ synthetic fibers such as nylon, PES or aramid, which are very ‘smooth’. As far as chemical bonds are concerned, Figures 8.69 and 8.70 show that, in the case of cellulosic fibers and possibly nylon, covalent bonds would be a possible explanation, given the fact that ‘simple’ condensation reactions between the methoxy groups of the resin and active H-atoms or amide groups in the polymer chain are possible.
In the case of PES or aramid, however, these explanations are not acceptable. These chemically resistant polymers have only a few free OH groups (PES) or no chemical bonds at all, which would make it possible to react with the resin under the prevailing conditions. A possible approach for the explanation of the adhesion — promoting effect of the predip is the formation of polyurethane structures on the
|
Figure 8.68 Microscopic section illustrating the thickness of a RFL layer (Aramid, dtex 840×1). |
surface of the fibers that may provide a diffusion mechanism due to similar solubility parameters [59].
8.10.6
 8 января, 2016
8 января, 2016  Pokraskin
Pokraskin 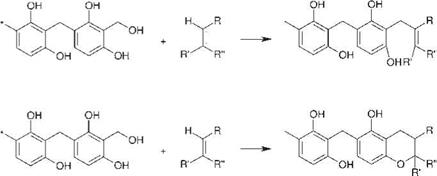

 Опубликовано в рубрике
Опубликовано в рубрике 