The skeptical engineer is wary of the idea that molecules leap into contact with their surrounding surfaces. After all, car parts on an assembly line do not suddenly jump together and adhere strongly to fashion the finished vehicle. However, at the molecular level it is quite obvious that such events occur naturally, and a nanoscale car could be self-assembled by these normal adhesive forces. The problem is that the nano-engine would not work because all its parts would seize together.
Proof of this is found in three simple experiments. The first experiment is the one interpreted by Thomas Young10 in 1805 and shown in Fig. 3.10. A droplet of water is lowered towards a plastic surface. As the drop approaches the polymer, very little happens until the gap between water and solid is extremely thin, around 1000 nm. This shows that the molecular adhesion forces do not extend far out from the surfaces. But at a certain separation, the water suddenly jumps onto the plastic and spreads rapidly across it. The final angle of contact is a measure of the molecular attractions, small angle indicating strong adhesion, large angle showing small adhesion.
In this example, it is clear that the water molecules are in constant motion, because small particles of pollen within the droplet can be seen dancing with the
|
Figure 3.10. Water droplet approaching a plastic surface, jumping onto the glassy material, and spreading over it. |
Brownian movement. Thus, the equilibrium of the droplet can be viewed as the rapid wetting and dewetting of the polymer surface by each individual molecule of water. If a force is applied to the drop, by tilting the plate, then the water can detach from the surface quite easily, while making new contact on the other side as the droplet rolls down the surface. Molecular adhesion at equilibrium is clearly only small in this instance, because the water does not strongly adhere to the polymer.
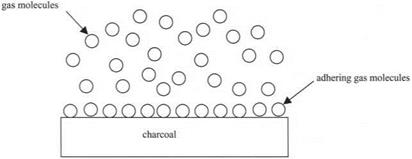
The second experiment showing molecular adhesion onto surfaces was described by Daniell in 1839.11 He took a piece of hot charcoal, which is composed of very fine strands of carbon with a large surface area, and showed how, on cooling, the charcoal would absorb large quantities of gas. He wrote of “a force of heterogeneous adhesion” which was causing the gas molecules to stick to the solid charcoal surface. The same effect can be observed in the famous BET12 apparatus in which a sample of porous material like charcoal is cleaned by heating to 200°C in a nitrogen gas stream, then cooled to liquid nitrogen temperatures (see Fig. 3.11). The nitrogen absorbs onto the charcoal, as can be seen from the reduction in pressure of the gas in the closed vessel. This is such a well-understood effect that it is used to measure the molecular surface area of powders by the number of nitrogen molecules adhering to the surface. In this case, the adhesion is again reversible and the gas molecules can be removed by heating the sample.
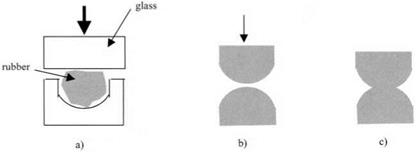
A third experiment which shows unequivocally that molecules leap into adhesive contact was performed by Johnson, Kendall and Roberts in 1970.3 This experiment was similar to Newton’s original test on glass telescope lenses (Fig. 3.1) but used rubber surfaces because they adhere much more reliably than glass. Roberts had developed a way of moulding rubber in concave glass lenses to produce remarkably smooth elastomeric spherical surfaces as shown in Fig. 3.12. The rubber composition was mixed and then pressed hot into the glass lens. After
|
Figure 3.11. Gas molecules adhering to a charcoal surface on cooling. |
|
Figure 3.12. (a) Moulding rubber lenses in glass formers; (b) bringing two rubber lenses together; (c) spreading of rubber to form a large contact spot. |
cooling, the rubber lenses could be peeled out of the moulds and then brought into contact (Fig. 3.12(b)).
As the two smooth spherical surfaces approached each other, within a few micrometers of contact, the familiar Newton’s ring pattern could be seen in the narrow gap between the smooth surfaces. Then, as the rubber lenses were moved still nearer, a sudden jump of the rubber was observed and the black contact spot grew rapidly to a large size as the rubber deformed and spread under the influence of molecular adhesion (Fig. 3.12(c)). The appearance of this was very similar to the liquid drop spreading over a glassy polymer surface. To get the rubber lenses apart, a tensile force had to be applied to overcome this molecular adhesion. It is this cracking apart of adhering surfaces which we consider next.
 9 сентября, 2015
9 сентября, 2015  Pokraskin
Pokraskin 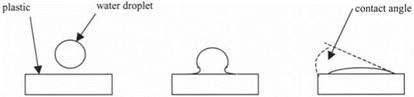
 Опубликовано в рубрике
Опубликовано в рубрике 