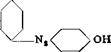
0. 1 mole (18.4 grams) of technical benzidine is tetrazotized as described on page 261. The clear tetrazo solution is poured rapidly into a solution of 15 grams of pure salicylic acid and 40 grams of soda ash in 300 cc. water at 5°C. The orange yellow intermediate compound separates, and the reaction is completed when a drop of the reaction mixture on filter paper gives, in the colorless ring, no blue coloration with alkaline H acid solution. The mixture is stirred gently until this test for tetrazobenzidine has disappeared, which requires about 1 hour at 12°.
* o-Tolidine is frequently used in combination with o-cresotinic acid, but rarely with salicylic acid because the resulting dyes are difficultly soluble.
The resulting diazo compound has the structure :
COOH
and can be reacted with various amines and phenols to produce important dyes. Thus, diamine brown M is formed by reacting the diazo compound with a carbonate solution of у acid. It is interesting that only 85 per cent of the theoretical amount of у acid is used in this preparation. If the intermediate diazo compound is treated with у acid in acetic acid solution, the important diamine fast red F is formed in the course of twelve hours at 12 to 28°C. The latter dye, because of its salicylic acid grouping, dyes chrome mordanted wool in shades which are fast to milling.
![]()
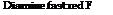
![]() . N,— Salicylic acid OH
. N,— Salicylic acid OH
n,—
HO’sVV
Diamine brown M
In alkaline solution, the diazo group enters the position ortho to the hydroxyl group, while in acetic acid solution, it enters the position ortho to the amino group.
Dianil Brown 3 GN
![]()
COOH OH
This dye is one of the most widely used direct azo dyes as it is an extraordinarily strong dye. It is not fast to acid or to light. The first step in its preparation involves the preparation of the monoazo dye, sulfo chrysoidine G:
|
H, N |
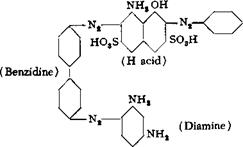
0.1 mole (17.3 grams) of sulfanilic acid is diazotized as described on page 261, and the suspension of the diazo compound, which must be weakly acid, is added slowly to 10.8 grams of pure m-phenylenedi — amine in 10 per cent solution containing 5 cc. concentrated hydrochloric acid. The coupling reaction is followed by testing the reaction mixture on filter paper with alkaline H acid solution, and the addition of the diazo compound is continued until a very weak, reddish color is formed in the test spot. At this point, the diamine has disappeared completely but azo dye formation has not yet occurred. The reaction mixture is held at 5°C. for 2 hours, and then sufficient 10 per cent soda ash solution is added to neutralize the mineral acid (about 6 grams of soda ash is required). After 3 hours, still at 5°, an additional 5.5 grams of soda ash is added, over a period of 1 hour, and the mixture is allowed to stand overnight. On the next day, 10 grams more of soda ash is added and the solution is allowed to stand for 3 hours. It is absolutely necessary to carry out this coupling of sulfanilic acid and the diamine under carefully controlled conditions or the final dye will be weak. Most of the sodium salt of the sulfo chrysoidine crystallizes out in the form of light reddish brown crystals. The total volume should be about 500 cc., but not much greater. This suspension is mixed at 10° with the intermediate compound from tetrazotized benzidine and salicylic acid (page 285), and the whole is stirred for 5 hours. The mixture is then warmed carefully to 30° and held at this point for 12 hours. It is then heated to boiling, and the dye is salted out with 200 grams of salt. The dye, which is a pure brown red in color, precipitates in an easily filterable form. The mother liquor contains a small amount of sulfo chrysoidine. The yield of dry dye is. about 95 grams. It dyes cotton uniformly only if it is mixed with 10 per cent of its weight of soda ash. The amount of soda used is quite critical just as in the case of direct deep black EW (page 292).
Technical Observations. If 1,2,4-toluylenediamine is used in place of m-phenylenediamine, an analogous dye is formed which is somewhat more fast to acids. In this case, one of the positions para to the amino group is occupied, and hence the formula given above for the m-phenylenediamine dye must be correct, i. c., the second azo group must enter the position between the two amino groups, and not the position para to the —NH2.[58]
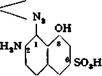
If the procedure is reversed, and the diamine is coupled first with the intermediate benzidine-salicyclic acid compound and then with diazotized sulfanilic acid, an isomeric dye, having the following structure, is formed:
|
COOH
|
Surprisingly, this dye is worthless.
It is very important to use pure diamine in this preparation since traces of o — or p-phenylenediamine destroy a large proportion of the diazosulfanilic acid and also of the benzidine-salicylic acid intermediate. The reaction mixture foams and tire dye is cloudy and weak. When pure materials are used, the yield of final dye is increased by as much as 40 per cent over that when technical diamine solutions are used.
Both of these dyes, that from m-phenylenediamine and that from toluylenedi — amine, are used in large quantities in the preparation of compound colors.
Diamine Green В
OH NHj
f ] ho, s^AJso, h
|
|
To the ice-cold diazo solution prepared from 14.5 grams* of pure p-nitroaniline according to the procedure on page 260, is added a cold solution of 34.1 grams of H acid and 5.5 grams of soda ash in 100 cc. water. The addition is made over a period of about 45 minutes, using thorough mechanical stirring so that no lumps are formed. The H acid combines with the diazotized p-nitroaniline in the course of 4 to 5 hours, forming one equivalent of hydrochloric acid. The mixture must
* This is used instead of the theoretically required 13.8 grams. This small excess is necessary because some of the diazo compound is decomposed during the long slow coupling reaction.
![]()
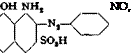 |
be allowed to stand for at least 12 hours and is then heated to 50°C. and treated with 20 grams of 30 per cent sodium hydroxide and 40 grams of soda ash. The monoazo dye, having the structure:
goes into solution with the formation of a blue color and is then salted out with 200 grams of salt. After a few hours, the sodium salt separates in an easily filterable form and is filtered off and pressed out. The mother liquor is deep blue in color but yields no usable dye when saturated with salt and is, therefore, discarded.
Instead of isolating the dye from p-nitroaniline, it can be coupled in soda solution at 5 °С. with the calculated quantity of diazotized aniline to produce the important dye, naplithol blue-black В (C), having the structure:
OH NH2
![]() -N.-A/VNi-Q NO,
-N.-A/VNi-Q NO,
HO, sl I Jso, H
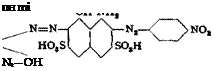
The monoazo dye is not isolated. An excess of diazotized aniline has a deleterious effect. The naphthol blue-black В is salted out at 90°C. with 15 per cent salt to yield a lustrous, bronzy product. This dye, on reduction with NaaS at 25°, yields a valuable dark green azo dye, azo dark green, having the structure:
The reduced dye is precipitated after 3 hours at 50° with 15 per cent salt and a small amount of sulfuric acid; it is difficultly soluble in bicarbonate. The mother liquor is deeply colored.
It is generally true that dyes from p-nitroaniline can be reduced practically quantitatively, by the calculated amount of sodium sulfide, to the p-phenylencdi — aminc dyes. The resulting aminoazo dyes can be diazotized, in turn, and combined with other couplers. The same aiuinoazo dyes are obtainable, however, by hydrolysis of the acetyl-p-phenylenedianiine dyes. (Formyl — and oxalyl-p-phenylenediamine can also be used. See page 95.)
4 X—N,—NO, + 7 H,0 + 6 Na, S
 |
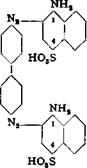
The sodium salt is dissolved at 80°C. in 500 cc. water containing 40 grams of soda ash, and the solution is cooled, with stirring, to 20°. Sufficient ice is then added to lower the temperature to 4°, whereupon part of the dye separates again in a finely divided form. To this suspension is added a solution of tetrazobenzidine, prepared as described on page 261, the addition being continued until a drop of the reaction mixture on filter paper gives a faint, but definite, blue ring with alkaline H acid solution. The coloration is not permanent at first, and more of the tetrazo solution must be added. About 18.6 grams of benzidine is used in all, and the formation of the intermediate compound requires about 30 minutes. The intermediate has the structure:
To this intermediate compound is added 12 grams of phenol, liquidised with a small amount of water, and the mixture is allowed to stand for 3 hours at 10°C., after which the temperature is raised slowly to 30° and held at this point overnight. The solution is then heated to 60°, and enough 30 per cent sodium hydroxide solution (about 40 grams) is added to dissolve all of the material. (Nitroazo dyes should not be treated with sodium hydroxide in the presence of wood or reducing materiab.) The solution is treated with 150 grams of salt, and dilute sulfuric acid is added in sufficient quantity to precipitate the dye completely (test by spotting on filter paper). The dye is then filtered off, pressed out, and dried at 90°. The yield is about 110 grams. Instead of using sodium hydroxide, the solution can be heated at 90° and the dye salted out of the hot solution with 300 grams of salt. The dye obtained in tins way is not so strong.
Technical Observations. In spite of its moderate light fastness, diamine green В is one of the most widely used green dyes for cotton. It is used for dyeing the insulation on telephone and other wire, and for the preparation of compound colors. When salicyclic acid is used in place of phenol, the first coupling must be done with the salicylic acid, since this compound does not react satisfactorily as the second coupler with benzidine. The dye formed in this case is diamine green G. This dye is less widely used since its preparation runs less smoothly, and the dye costs more as a result. In the industry, heating is always done by blowing in steam and the resulting dyes cannot be pressed out because they pass through the filter cloths.
|
Direct Deep Black EW
|
In the preparation of diamine green B, we have seen how H acid, in mineral acid solution, couples very easily with p-nitroaniline to form a monoazo dye in which the azo group enters the position ortho to the amino group. Benzidine, however, couples appreciably less rapidly, and it is necessary to neutralize the free mineral acid that is formed. Contrary to the statements in the patent literature, it is not possible to carry out this reaction in acetic acid solution, because H acid, in the presence of sodium acetate, couples immediately in the position ortho to the hydroxyl group. These facts have given rise to many patent suits, all of which, however, have been decided in favor of the holders of the mineral acid coupling patents.
|
(a) The Intermediate
|
Following the procedure given on page 261,19.2 grams of benzidine is tetrazotized, and the temperature of the resulting solution is adjusted to 10-12°C. To the tetrazo solution is added, over a period of 1 hour, the filtered solution of 34.1 grams of H acid in 300 cc. water containing
5.5 grams of soda ash. The H acid solution should be distinctly acid to litmus. The mixture is stirred for 3 hours at 12°, and then a solution of
5.5 grams of soda in 60 cc. water is added very carefully over a period of 2 hours, the addition being made in such a way that the reaction mixture retains a mineral acid reaction at all times. After an additional 3 hours at 12°, the reaction mixture is brought to a point where it is
just faintly acid to Congo red by the addition of a more dilute solution of soda and allowed to stand in a cool place overnight. After 12 hours, the intermediate compound has separated completely as a powdery precipitate, and the mixture gives no test for tetrazobenzidine (with H acid solution) or for H acid (with diazotized p-nitroaniline).
|
(6) The Intermediate
|
The diazonium solution from 8.8 grams of pure aniline, prepared as described on page 259, is added to the product from (a) at 5°C. If necessary, ice is added and then a solution of 26 grams of soda ash in 120 cc. cold water is poured in with thorough stirring. A clear solution is formed momentarily, and then the new intermediate compound separates. If too much soda is used, there is danger of the intermediate coupling with itself. The coupling reaction can easily be followed by spot tests on filter paper. Frequently, the test for diazobenzene does not disappear completely.
After about 15 minutes, 11 grams of pure m-phenylenediamine, dissolved in a small amount of water, is added to this second intermediate compound. Coupling proceeds rapidly, and part of the dye goes into solution. After 1 hour at 14°C-, the mixture is carefully warmed to 50°, and 10 grams of soda ash is added. It is then treated with 120 grams of salt and acidified, with continuous stirring, by the addition of about 20 cc. concentrated hydrochloric acid to precipitate the dye. The dye is insoluble at 50° in 10 per cent salt solution containing bicarbonate, provided the solution has not previously been boiled. The product is very easily filterable, and can be dried at 100° after being pressed out. The yield is about 100 grams. The dye does not go on cotton properly unless it is mixed with 6 per cent of its weight of soda.
If m-toluylenediamine is used in place of m-phenylenediamine, deep black V is formed. This dye gives somewhat more reddish shades. In this case also, it is necessary to add some soda after warming the reaction mixture in order to obtain a filterable product.
Technical Observations. The cotton black described above is the most widely used direct black in the dye industry. It is used in dyeing all organic materials, such as cotton, half-wool, leather, etc. The dye is prepared in the largest azo dye installations, and only the purest starting inateriak are used. The best yields and the highest quality of product are obtained when m-phenylenediamine, recrystallized from water, is used.
|
Congo Red
|
A solution, prepared by tetrazotizing 18.4 grams of technical benzidine (page 261), is mixed with a solution containing 50 grams of naph — thionate and 50 grams of sodium acetate in 200 cc. water. The temperature is held at 5°C. for 1 hour, then raised slowly to 20° where it is held for 5 hours. Subsequently, the mixture is stirred at 30° for 24 hours, and then, on the third day, is heated to 55°. After the coupling has proceeded for 2.5 days, the reaction mixture is heated to boiling and treated with 40 grams of calcined magnesia, whereupon the insoluble magnesium salt of Congo red is precipitated. The salt is filtered off and washed thoroughly, thus removing all of the impurities. The washed magnesium salt is stirred into 500 cc. boiling water and the mixture is treated with 15 grams of soda ash, which precipitates the magnesium as the carbonate and dissolves the dye as the sodium salt. The hot solution is filtered, washing the magnesite with water, and the Congo red is salted out by adding 15 per cent (by volume) of salt to the filtrate. The dye is precipitated as a light red solid which, after drying, weighs about 70 grams.
Technical Observations. Congo red, the first of the benzidine dyes, is widely used despite its sensitivity to acids. It is not surpassed in beauty by any other direct dye. Congo red is manufactured by only a few firms because its low price does not allow for much margin of profit.
In large scale preparations, the coupling reaction is often carried out somewhat differently. The reaction is greatly accelerated by adding the naphthionate solution at 85°C. to the tetrazobenzidine solution. Very thorough stirring is required under
these conditions, and the operation is carried out in small batches. 8 or 10 runs can be made in one day, however. The excess naphthionate is often recovered.
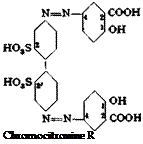
In addition to Congo red, benzopurpurin 4 B, prepared from o-tolidine and naphthionic acid, is also of importance. In the preparation of this dye, the coupling reaction cannot be carried out at higher temperatures since the tetrazo compound from o-tolidine is too unstable. Benzopurpurin 4 В is somewhat less sensitive to acids than Congo red, and, like the latter, is widely used, particularly in the Orient. It is interesting that these dyes are found to be much more stable in nonindustrialized countries where the atmosphere contains little sulfuric and sulfurous acids.
 |
 |
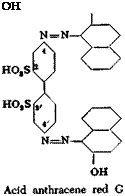
Acid Anthracene Red G and Chromocitronine R
Benzidinedisulfonic acid is so insoluble that it must be diazotized indirectly by dissolving it in soda or caustic solution, adding nitrite, and adding the solution to acid.
Acid Anthracine Red G. A solution is prepared by warming 34.4 grams of benzidine-2,2′-disulfonic acid (100 per cent) with 300 cc. water containing 11 grams of soda ash. The solution is cooled to 20°C., treated with 14 grams of sodium nitrite, and is added to a mixture of 60 cc. 30 per cent hydrochloric acid, 200 cc. water, and 100 grams of ice. The temperature can go to 25° without damage, and the diazotization is complete within a few minutes. The tetrazo solution is added to a solution of 30 grams of /J-naphthol prepared by using the same proportions of water, sodium hydroxide, soda, and ice as were used in the preparation of acid orange A (page 262). The reaction mixture is worked up much as in the case of acid orange A. Sometimes, however, the tetrazobenzidinedisulfonic acid separates out as an insoluble, coarsely granular, crystalline precipitate which does not react with the alkaline
naphthol solution. In this case, it is necessary to treat the ice-cold tetrazo solution with enough sodium hydroxide to form the soluble sodium diazotate which couples instantaneously with the /3-naphthol. Acid anthracene red G gives shades on unmordanted wool which are fast to milling.
Chromocitronine R. The solution of tetrazotized benzidinedisul — fonic acid is added at 5°C. to a solution of 32 grams of pure salicylic acid in 200 cc. water containing 80 grams of soda ash. After 12 hours, the resulting dye is salted out in the cold by adding 20 per cent of salt (based on volume). It is then pressed out, and dried at 60°.
In this case, it is unnecessary to redissolve the tetrazo compound which may separate out under certain conditions, because the chromocitronine itself dissolves immediately. It is advisable, however, especially in large scale preparations, to filter the final dye solution before the salting out operation so that the product does not cause fouling of the printing roll in calico printing where the dye is widely used. The wooden vats frequently produce splinters causing much trouble to the calico printer. Chromocitronine R is a beautiful yellow dye whose chromium lake has exceptionally high fastness to light, washing, and chlorine. Because of its high solubility, the dye penetrates deeply into cotton, giving the appearance, on thin materials, of being printed on both sides.
 3 декабря, 2015
3 декабря, 2015  Pokraskin
Pokraskin 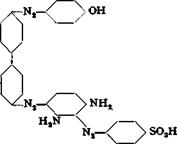
 Опубликовано в рубрике
Опубликовано в рубрике 