In order to produce a waterborne epoxy coating, the resin must be made compatible with water. There are many methods used in achieving this and some are:
• emulsify the resin directly in water using an emulsifying surfactant or a water soluble polymer to stabilise the system
• use either an acid or a base functionality on the epoxy to product an ion compatible resin which can then be dispersed in water.
However, waterborne polymers used in interior can coating lacquers over the past fifteen years, have generally been established upon base neutralised, acid modified epoxies, in conjunction with acrylic functionality. The neutralisation of the epoxy generates a water compatible resin, the acrylic portion tends to lend adaptability to the resin. Changing the monomers in the acrylic component can produce significant changes in the lacquer properties, e. g. additional reaction with the epoxy component, greater film flexibility, better substrate compatibility.
Typically, a solid epoxy of 3000 to 4000 EEW (Epikote 1007 or 1009 types or an analogue material manufactured by the chain extension of a lower Mw liquid epoxy resin) is modified to provide an acid functional epoxy. In general, the acid functionality can be conferred by two methods, acid capping (see resin 1 and resin 2) of the oxirane groups or by the graft polymerisation of an epoxy with a carbonyl functional co-polymer (see resin 3). The co-polymer can consist of the reaction product of a free radical polymerisation of any approved ethylenic unsaturated monomers containing carbon-carbon unsaturation, e. g. carboxyl functional acrylic monomers, (acrylic acid, methacrylic acid, etc.), the lower alkyl esters, vinyl monomers (acrylamides), vinyl esters (vinyl acetate, vinyl butyrate), vinyl aromatic monomers (styrene, a methylstyrene) etc. The acrylic ‘capping’ resin is acid functional, being based upon either methacrylic or acrylic acid. The former is normally preferred. An acid value of 50-100 mg KOH/g would be typical.
The following is an example of a typical formula and procedure used in manufacturing a (8,phosphatised epoxy resin for use in waterborne can coating lacquers.
|
FORMULATION 2-17: PHOSPHATED EPOXY ACRYLIC RESIN (RESIN 1)
|
Non-Volatile Content 43 ± 1% @ 193°C for 10 minutes
The epoxy is dissolved in the BDG at 122°C to produce an epoxy solution, the concentrated (85%) phosphoric acid is then added under agitation, then a further addition of BDG is added. Then mixture is agitated and held at 123°C over a period of 30 minutes, cooled to 121 °С, and DIW (deionised water) is added over a period of 10 minutes. The mixture is held under agitation at 124°C for two hours, then the acrylic co-polymer(s) are mixed in over a period of 30 minutes at a temperature of ~124°C. Finally the mixture is reduced in viscosity and a water compatible dispersion is produced, with the addition of the amine mixture. It should be noted the co-polymer should be added in the correct order otherwise the dispersion will prove to be unstable.
Below is an example of a typical formula and procedure used in manufacturing*9′ a benzoic acid capped epoxy resin for use in a waterborne can coating lacquer.
|
FORMULATION 2-18: BENZOIC ACID CAPPED EPOXY ACRYLIC RESIN (RESIN 2)
|
Non-Volatile Content 28 ± 1% @ 193°C for 10 minutes
The liquid epoxy, diphenylolpropane, benzoic acid and butyl glycol are mixed together, heated to ~130°C, allowed to exotherm to 200°C, then held at 165°C for two hours after the peak exotherm. The resulting reaction product is then cooled, with the addition of n-butanol to ~100°C. The acrylic co-polymer and DIW are mixed together and held under reflux at ~95°C for twenty five minutes. The acrylic resin solution is added to the acid capped epoxy under agitation at ~70-80°C over a period of approximately one hour. The resulting aqueous dispersion can be neutralised with a tertiary amine and used to formulate can coating lacquers.
The following is a typical example of an (10)epoxy-acrylic graft co-polymer used in waterborne epoxy can coating lacquers.
|
FORMULATION 2-19:
*DER is the trademark of Dow Chernies Properties |
Non-Volatile Content 25 ± 1% @ 193°C for 10 minutes
The epoxy chain extension reaction is normally carried out under an inert, dry, nitrogen blanket. The liquid epoxy is heated to 80°C, then diphenylolpropane is added and mixed in. The mixture is heated to ~191°C over two hours and held at that temperature under agitation for a further two hours until an epoxy of EEW 3500 — 4000 is produced, then n-butanol and butyl glycol are added. The acrylic monomers are then mixed together, and added gradually over a period of two hours to the epoxy solution held at a temperature of 118°C. The resulting resin is then cooled to 85°C, and dropped into a mixture of DIW, and TEA under agitation. DMAE is the preferred alternative.
The monomers are added to the solution of epoxy in order that a free radical initiated polymerisation can take place in the presence of the epoxy. The grafting of the addition polymer onto the aliphatic carbons of the epoxy, takes place during this polymerisation stage.
Acrylics are used to form epoxy-acrylic graft copolymers by hydrogen abstraction from the epoxy backbone. The introduction of carboxylic acid groups, through the use of carboxy functional acrylic monomer in graft co-polymerisation at these epoxy backbone sites is the mechanism by which the epoxy is rendered water dispersible. See Figures 2-20 and 2-21. The pendant carboxylic acid groups can be neutralised with amine, introducing the ionic character that is required to stabilise the system in an aqueous media, through the formation of micelles.
 |
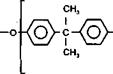 |
Graft Sites
Figure 2-20
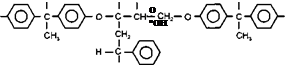 |
||
Grafting Sites on Epoxy Resin Backbone
Figure 2-21
Epoxy Acrylic Graft Structure
Not every molecule of epoxy present during the polymerisation is modified by reaction with the acrylic, as shown in Figure 2-22. Some epoxy is left unmodified and some acid functional acrylic resin is formed, which is not grafted onto an epoxy backbone. The molecules of epoxy which are grafted with acrylic perform an important function as they contain both hydrophobic and hydrophilic parts on them, which when neutralised with amine, give them the classical features of an emulsion stabiliser. They can be drawn with the ionised carboxylic acid groups on pendant side chains attached to the main epoxy backbone as shown in Figure 2-23.
This kind of structure is described as a ‘comb’ stabiliser. The hydrophilic carboxylic ions arrange themselves around the water interface of the micelle particles. The hydrophobic, unmodified epoxy, is encapsulated on the inside. The characteristic milkiness of these water dispersed systems is caused by the light scattering of these particles. In fact, the characteristic blue haze of these dispersions is a function of the particle size distribution.
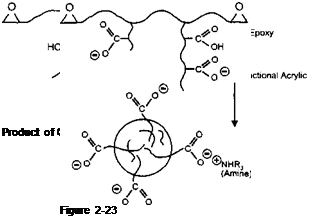 |
A good sub-micron particle size distribution will display this blue haze. Another very important feature of the stabilisation of these particles is that the carboxy anions are chemically attached through non hydrolysable bonds to the particle, rather than just physically associated with the surface. This endows the system with good shear stability. Remember that these lacquers are sprayed under high pressure onto the inside of the can. During this operation, the particles are subjected to heat and a considerable amount of turbulence. The fact that the stabilising group is chemically attached to the particle means that it is robust enough not to get knocked off. The consequences of the stabilising groups being knocked off would be quite dramatic for the user, as the particles would coagulate and block filters.
The free carboxyl groups on the acrylic graft co-polymer can be neutralised with a tertiary amine which can activate an esterification reaction between the acrylic carboxyl moieties and the epoxy reactive sites. On the addition of de-ionised water, the free carboxyl moieties of the acrylic co-polymer react with the amine to produce a water soluble or dispersible quaternary ammonium hydrogel of the modified epoxy.
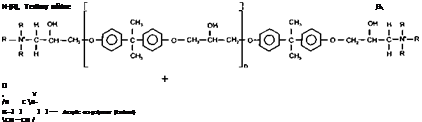 |
The quaternary reaction takes the following form shown in Figure 2-24.
Figure 2-24
Quaternisation of an Epoxy Graft Acrylic with Tertiary Amine
1. Hahn K., Ley G., Schuler H., Oberthur R., Colloid Polymer Sci. 264,1092 (1986)
2. Kowalski A., Vogel M., Polenkeinship J., European Patent 22,633 (1980)
3. European Patent 0256391 to Glidden
4. US Patent 5201436 to Glidden
5. Woo J. T.K., Tiny V., Evans J., Ortiz C., Carlson G., Mocinko R., ACS Symposium Ser 221 (1983) p283
6. Clarke J., Surface Coatings International 7, 303-305 (1994)
7. Winnik M. A., Wang Y., Haley F., Latex Formulation at the Molecule Level. Journal of Coating Tech. 64 No. 811, 51(1992)
8. US Patent 4638020 to PPG
9. US Patent 4302373 to Du Pont
10. US Patent 4212718 to SCM

 26 июля, 2015
26 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 