Ideally, conversion will keep pace with the feed rate and at t, there will be complete conversion of monomer to polymer. In practice, the conversion will lag a little behind the addition rate, the reaction not being complete until time t2.
It is usual, when conducting commercial polymerisations, to monitor the reaction profile against a predetermined norm. Because the reactants are being continually renewed at a rate close to the rate of consumption, the copolymer composition tends to be more uniform. Since the ratio of initiator to monomer remains more constant than that which is experienced with an “all-in” process, a much narrower molecular weight distribution results.
A method sometimes used commercially is to combine the two techniques. An initial aliquot of monomer and initiator, normally up to 25% of the total charge, is added to the reaction vessel, together with the initial solvent charge.
The mixture is heated to reaction temperature and, after a suitable period, the remaining monomer and initiator are fed to the vessel at a predetermined rate. The molecular weight distribution obtained with this technique is much wider than that obtained by using a “drip feed” process alone.
The process is illustrated graphically below where:
to represents the point at which reaction temperature is reached ti represents the point at which the feed of the remaining monomer begins U represents the point at which the feed is complete.
The practical curve should, ideally, parallel the theoretical curve indicating no build up of free monomer.
|
TIME ———- ► Figure 1 -68 |
 8 июля, 2015
8 июля, 2015  Malyar
Malyar 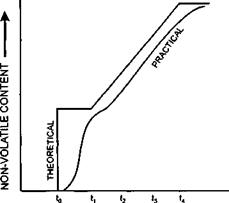
 Опубликовано в рубрике
Опубликовано в рубрике 