Polymers for electrodeposition are formulated as polyelectrolytes. That is to say that they are made containing a proportion of functional groups which, when reacted with an acid or a base, as appropriate, will have a multiplicity of ionic groups along the chain. These ionic groups make the polymer compatible with water. In some cases the polymer is essentially dissolved in the water, and the solution is clear. In other cases a proportion of a suitable organic solvent is needed to produce a clear solution, and in still other cases the polymer is there essentially as a dispersion and the solution is opaque. These opaque solutions perform perfectly well and a clear solution is not necessary for electrodeposition (or for any other aqueous paint system).
The polymers can be divided into two types, depending on which electrode they will deposit onto. Thus resins which deposit on the anode are known as ‘anodic’ resins, and those which deposit on the cathode are known as ‘cathodic’ resins.
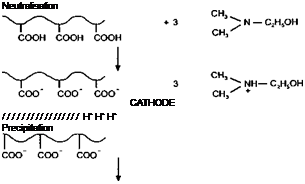
Anodic resins are those which contain acid groups along the chain and which are made water dispersible by the addition of a base. The acid-base reaction produces carboxyl ions (COO ) along the chain, stabilising the aqueous dispersion.
Cathodic resins have basic groups along the chain and these can, of course, be any of a number of different organic groups. The most usual are amine groups and primary, secondary and tertiary are all used. Most practical formulations contain tertiary amino groups but this is not universal. Other groups such as phosphonium and sulphonium groups appear from time to time in the patent literature but are probably little used, if at all, in commercial products.
As can be deduced from the discussion in the previous section, the processes that occur during electropainting give rise to an accumulation of hydrogen ions at the anode and hydroxyl ions at the cathode. If a polymer which is stabilised by the existence of carboxyl ions along the chain approached the anode, the carboxyl ions will be neutralised and the polymer will precipitate out of solution onto the anode. Similarly, if a polymer stabilised by acid-neutralised amino groups approaches the cathode, then this will be precipitated out by the hydroxyl ions.
A certain minimum concentration of the hydrogen of hydroxyl ions is necessary to actually precipitate the resin, which means that it does not begin to precipitate until a certain minimum amount of electricity has passed. All the time the current is passing the ions are diffusing away from the electrode by normal diffusion processes, so if the current passing is not high enough to overcome this, the resin does not precipitate — it has been found experimentally that there is a minimum current below which no film is formed.
The actual amount of electricity which needs to flow before the resin begins to precipitate depends on how soluble (or insoluble) the resin is, and on how much acid or alkali has been used to neutralise it.
Figures 2-12 and 2-13 show, diagrammatically, typical reactions for anodic and cathodic resins. These are, of course, only diagrammatic and anodic resins will, of course, contain more than the three groups shown, and cathodic resins will not normally contain all three types of functional group.
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
![]()
![]()
Figure 2-13
Reactions of Cathodic Electropaints
This, then, is the essence of the electrodeposition process. To be successful the polymer must be soluble when ionised, and insoluble when not ionised. This is why quaternary ammonium ions do not produce a cathodic resin because the fourth organic group cannot be removed by merely changing the pH, as can the hydrogen of a neutralised primary, secondary or tertiary amine group.
Before going on to discuss other aspects of the process, it is worthwhile talking about how the polymer ions arrive at the electrode. Many people believe that they are brought there by the action of the electric field, but the mobility of the average polyelectrolyte is so low, because of its large size, that this is a very minor occurrence. The polymers arrive at the electrode because of the agitation of the solution, together with diffusion across the double layer.
The electropainting process is frequently described as ‘electrophoresis’. This is a misconception which arose in the very early days when nobody knew quite what was happening, and has never been erased since. Electrophoresis is an entirely different process involving the movement of ions in an electric field and is much used in, for example, the analysis of proteins. It is possible to coat an electrode electrophoretically, but this is done under different conditions than those used in electropainting.
The pigments, and non-ionic resins such as melamine-formaldehyde crosslinkers, are held in suspension by the ionic polymer, and precipitate with it. They do not always deposit on the electrode in the same ratio that they are present in the original paint, and this is a factor which has to be determined experimentally for each system. When a difference is found this can be allowed for by adjusting the relative concentrations in the formulation.
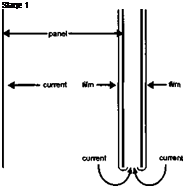
Consider what happens whilst the film is being deposited on the electrode. The deposited film forms a high resistance barrier which reduces the current density at that place on the electrode. If there are other portions of the electrode which are not covered with a film, then the current will continue to pass there and they in turn will be covered with a film of deposited paint. This will continue until all the electrode is covered with a film, except those parts which are so remote from the counter electrode that their drop is as high or higher than the voltage drop caused by the film resistance. Over most of the work, therefore, the film is of uniform thickness. The ability to coat remote parts of the job with an acceptable film thickness is known as ‘throwing power’, or sometimes ‘throw-power’, and is discussed more fully below. A diagrammatic representation of the early stages in the deposition process is shown in Figure 2-14.
major
current
flow
/’
 |
 |
Start Stage 1
Figure 2-14
Stages in the Electrocoating Process
Current continues to flow after the electrode has become covered with a film and conduction is largely through the film. The current density thus becomes dependent mainly on the film conductivity, as this is so much lower than the solution conductivity. The current continues to fall as the film thickness builds up and so, in consequence, does the increase in film thickness. This gives a practical limit to which the film can be deposited in a given set of circumstances. A greater rate of deposition and a greater film thickness can be achieved by increasing the voltage and/or the film conductivity but the extent to which this can be done is limited by the phenomenon of ‘rupture’, discussed below.
As explained in the previous section, gas is evolved at the electrode from the electrolysis reaction. The amount is comparatively small (about lcc for each 4 coulombs passed) and this escapes through small holes in the film. The film must therefore be sufficiently liquid to flow into the holes afterwards, or else the final film will be full of pinholes. The extent of gas-bubble polarisation is probably very small.
The concept of ‘throwing power’ is illustrated in Figure 2-15, which shows diagrammatically a typical cell used for measuring throwing power. This consists of two steel panels, 4 inches by 12 inches, which are held at a distance of about 1/4" apart by two non-conducting spacers along the long edge, thus forming a hollow box 12" long, 4"
wide and 1/4" deep, open at the end. This is immersed in the bath of paint, open end downwards, this is a standardised arrangement which mimics the sort of situation which occurs when painting a box-section on a car body. This is then electrodeposited with the test paint, and the distance to which the paint reaches on the inside of the box is a measure of the throwing power of the paint.
![]()
 Stage 2
Stage 2
▲
I I
very
low
current
Figure 2-15
Throwing Powder
To enable the resin to be precipitated, a minimum current must pass and in the initial stages this is governed by the resistance of the electrolyte. The current density is therefore highest at those parts of the test panels closest to the counter electrode, and the paint will begin to deposit there first. Once a film of paint has begun to form on the portions of the electrode with the highest current density, then this increases the resistance at that point, so the current falls, and more current can flow at the portions of the electrode further away. The coated portion thus increases in size and the electrode becomes covered with paint. At the portion of the panel where there is only a small gap, the solution resistance is much higher (because of the smaller cross section area) and so the current is smaller. At some point in the slot the current will be below the minimum deposition current, and no paint will be deposited. If the applied voltage is increased, then a greater length can be coated, but the voltage which can be applied is limited by the film thickness wanted on the outside, the rupture voltage of the film, and the voltage available at the power pack.
As mentioned above, if the voltage is increased too much, or the film conductivity increased too much, then a phenomenon known as ‘rupture’ occurs. This is characterised
by a large increase in current density (usually over a limited area of the film) which results in an unsightly area of rough thick film. The cause of this has been shown to be the heat generated in the film being sufficient to boil the residual water in the film, thus disrupting the film integrity and allowing more current to pass.
In practice, rupture is found to occur at a particular voltage and the ‘rupture voltage’ is one of the important characteristics of a paint formulation. Typical values of the rupture voltage vary from about 50 volts for the older alkyd type resins, to over 400 volts for the new cathodic primers and high-throw acrylics. Without going into the theory too deeply, it can be said that the major factor controlling the rupture voltage is the film resistance. It is an important practical problem and it is worthwhile discussing the factors which affect the film resistance
We can do this by remembering that the conduction through the film is still ionic, and that therefore the ions carrying the current must pass through the film. The most mobile of the ions present are the hydrogen and hydroxyl ions and these will be expected to carry the greater part of the current. The mobility of these two ions is so much higher than other ions that it has been conjectured that the current is not carried by the physical movement of the ions themselves as in other bases, but by a shift of the charge along the hydrogen-bonded structure of liquid water (which is a highly structured liquid).
The conductivity of our film therefore, will be highly dependent on the amount of water in the film and on the presence of other groups which are either hydrogen donors or hydrogen acceptors and which can therefore contribute to the highly hydrogen-bonded structure which gives the hydrogen and hydroxyl ions their high mobility.
The two elements likely to be present with a high level of hydrogen-bonding capabihty are oxygen and nitrogen, so that the presence of hydroxyl, carboxyl, ester, ether, amine, and amide groups can be expected to lower the film resistance. All these groups are, in addition, hydrophilic and will contribute to the presence of more water in the film by their presence. It is essential to have a certain minimum of some of these groups, of course, in order to provide the water solubility and crosslinking capabihty.
Hydrocarbon groups have the opposite effect because they are hydrophobic so a lot of hydrocarbon (as, for example, in a polybutadiene resin) will help in increasing the film resistance. This cannot be taken too far. Too much hydrocarbon will make the emulsion unstable, thus it will be impossible to produce a practical system. Thus, a balance needs to be found between the hydrophilic and hydrophobic portions of the polymer molecule.
The actual resin, however, is not the only thing that contributes to the film resistance. Other constituents of the paint also have their effect. Thus, it has been well known for many years that solvent dissolved in the film reduces the film resistance. This can sometimes be a benefit, because it gives a higher film weight for the same applied voltage, or it can be a disadvantage because it lowers the rupture voltage and the throwing power. The effect would appear to be a result of the lowering of the viscosity of the film, thus increasing the ionic mobility because a hydrocarbon solvent such as xylene has a strong effect.
The pigment also has an effect and higher pigment-binder ratios give rise to a higher film resistance. Laminar pigments such as talc or red oxide have a greater effect in this respect than more crystalline ones such as titanium dioxide.
 22 июля, 2015
22 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 