The preceding formulation guidelines will on occasion appear to contradict themselves. pH value recommendations, for example, fluctuate wildly depending upon the need to prevent flash rusting, accommodate an aluminium pigment or prevent flocculation in a black. As with many formulating requirements in the coatings industry, the final result is a compromise, reached empirically rather than theoretically.
Although a vast number of applications for waterborne coatings have been discovered, their usage is not as widespread as originally expected or desired. The major reasons for this are the special technical considerations and the overall cost of going “green”. However, failure to acknowledge the importance of waterborne polymers is a grave mistake. Polymer development is proceeding at such a fast rate that it is difficult to predict the point at which it will finally slow down. The introduction of self-crosslinking copolymer types gives the resin formulator yet more scope to achieve the high molecular weight polymer network the coating formulator requires. Their value with regard to environmental protection and industrial safety is beyond question. With a little end user re-education and process modification, waterborne coatings will find widespread acceptance.
The decorative paint market was the first major application for vinyl and acrylic emulsion resins. Over the last 30 years, waterbased emulsion paints have virtually replaced solvent based alkyd paints for decorating walls and ceilings and are gradually making inroads into the gloss paint market for doors and window frames etc. Eventually this market will have a large proportion of vinyl and acrylic emulsion based paints, particularly acrylic based emulsion paints for more demanding architectural applications. It is in the architectural decorative paint market that thermoplastic acrylic based emulsions are mainly used. For most industrial applications, even the high molecular weights obtainable by emulsion polymerisation are inadequate to give the required levels of film performance. Thermoset systems are needed for optimum resistance properties and mechanical performance.
Whilst there are some thermoset acrylic emulsions commercially available, the bulk of the thermoset resins, used as the main binder system, are produced in solution. Some may then be made waterborne by neutralisation and inversion (dispersion) into a water phase. Lower molecular weights favour this approach. The rate of conversion from solvent based to waterborne industrial thermoset coating systems has been, and is, much slower than the conversion from architectural alkyd paints to emulsion alternatives. There are two principle reasons for this. Firstly there are problems of application and substrate wetting of many waterborne systems. Secondly, the modifications frequently required to induce water dispersibility reduce one or more of the essential performance properties required from the cured film, compared to a solvent based system. Water resistance, with many films having an increased tendency for blushing is one example. However, for some applications, such as electrodeposition, only waterborne systems will work.
![]()
|
|
|
|
|
|
|
TABLE 7-12: SOME WAYS OF MAKING ACRYLIC RESINS MORE
ENVIRONMENTALLY FRIENDLY
 |
As can be seen, there is more than one route for making acrylic resins for surface coatings more environmentally friendly and they are not all waterborne. This theme will be returned to later in this section. Some aspects of VOC reduction will be briefly considered to demonstrate that there is not one clear solution for the coating’s industry.
The VOC reduction emphasis world-wide is very commendable. But one should question the rational behind taking it to the ultimate limit. Most countries in the Western world are committed to reducing VOC’s by 60-70%, by the year 2001 at the latest. In 1990 solvents accounted for 30% of the total anthropogenic, non-methane VOC emissions in Europe [Bouscaren 1990]. The surface coating industry accounted for 36% of the solvent emissions, making this industry’s emissions less than 11% of the total European VOC’s. Petrol and diesel emissions are one of, if not, the largest source of VOC’s.
With VOC reduction there is a law of diminishing returns, because if none of the solvent were replaced by water then a 40% nvc coating would have 60% organic solvent and a VOC of 1500 g/kg. Surely a reduction from 60% to 14% solvent is very commendable. The additional problems associated with going from 14 to 10 or 10 to 5% solvent are arguably not worth the return. In many instances this ‘solvent’ not only consists of organic water-soluble solvents, but amines used to induce water dispersibility to acid rich resins by neutralisation prior to inversion in water. The amine contributes to the VOC of a coating and depending upon the acid value of the resin and the type of amine the VOC from the amine can easily be in excess of 50 g/kg.
Reducing solvent increases wetting problems and the environment can suffer by more severe substrate pretreatments needed to ensure adequate wetting and flow-out. Waterbased systems cannot be disposed of by tipping them ‘down the drain’. Specialist effluent treatment is required. Water is a misnomer in this instance.
Water does not necessarily mean cheaper either. Too many end users believe that because water is less expensive than organic solvents, a waterbased coating should be less expensive than a corresponding solvent based one. To induce water solubility whilst retaining the desired performance characteristics, can result in more expensive raw materials being required and often lengthier and more costly processing.
The resin and coatings industries of Europe and North America should be proud of their achievements. Reducing VOC’s to the extent that has happened and is going to happen is highly commendable and it is not receiving the good publicity and praise that it deserves. Unfortunately the chemical industry is second only to the nuclear industry in unpopularity and is considered by many to be responsible for the deleterious effects on the modem quality of life. It receives much bad publicity, mostly unwarranted. Like it or not, surface coatings are part of this undesirable chemical industry. We as an industry have not been the best in the past at public relations, much to our current detriment.
There are, of course, geographic and regional variations to the overall VOC reductions. Some countries lag way behind others. Northern Europe as a general rule is ahead of Southern Europe. Europe and North America are far ahead of China and some developing African and Asian countries. The pressure for VOC reductions needs, in the editor’s opinion, to be focused towards those countries where little progress is being made. Arguably reducing the organic solvent from 14 to 10% in surface coatings in Europe and North America will have less benefit globally than reducing it globally from 100 to 50% in the developing countries. Economically the industrialised Western World is bearing the cost of environmentally lead legislation. Whilst this is as it should be, it is arguable if there will come a point in time when the Western World is price disadvantaged and uncompetetive compared to some of the developing countries.
The limit on total emission of organic material, once set, will create a new set of problems as pressure on increased productivity forces VOC reductions. Some of these restrictions are outlined in Table 7-13.
TABLE 7-13: EFFECTS OF RESTRICTIONS ON TOTAL ORGANIC EMISSIONS
Limited production of the number of coated articles
results in either:
a) more efficient application per metre squared coated — hence less wastage
or
b) further VOC reductions so that more articles can be coated for a given total emission
or
c) application of thinner films
In some factories in North America, the drive for lower VOC’s is to enable them to coat more articles per day. They may work on a bubble concept, which controls the total organic emissions from the factory. The number of gallons of coating purchased and its VOC are known, thus the only way more coating can be used is to reduce the VOC of the coating. If the VOC limit is exceeded, then it is possible production would cease until the end of the period for that VOC bubble. There are some other possible ways of increasing the number of articles coated without reducing the VOC of the coating and they have been given in Table 7-12. However, the simplest solution for the user of the coating is for them to request lower and ever lower VOC coatings.
Associated with VOC’s is the question of odour. People do not like smells they do not understand. They readily accept farmyard smells if they are in the countryside, but they will not accept chemical smells near a coatings factory, whether the smells originate there or not.
Odour can be considered to arise from many sources. Some of these are summarised in Table 7-14.
TABLE 7-14: SOME SOURCES OF ODOUR GENERATION
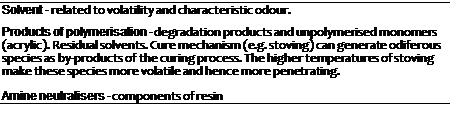 |
The method of application can have some bearing on how odiforous a particular process can be. It should be borne in mind that some of the raw materials used for some of these alternative application routes may be inherently less odiferous. Certainly reducing the amount of solvent will reduce the potential for causing odour. Of course, not all articles can be coated by all of these techniques. An approximation for a general comparison of odour to method of application is given in Table 7-15.
|
TABLE 7-15: ODOUR RANKING (Courtesy B. L. Hitchins, Coates Coatings UK Ltd.)
|
It is surprising to many that the method of application can have such a large effect on odour. Even with waterborne systems, incineration may still be required to reduce or remove odour. In this instance, it may not be economically viable to use waterborne systems, because solvents have calorific values which means that they can fuel or at least partially fuel an incinerator, thereby reducing the need for expensive fuel oil. It may be that a smaller incinerator can be used with waterborne systems compared to a solvent based system, with associated lower capital costs. The overall comparisons are not straightforward and ‘all’ factors must be considered in the overall cost equation.
From the preceding discussions it should be clear that waterborne systems will not replace all other coating systems in the foreseeable future. There will be a place for every technology, with some dominating. Solvent based systems will continue to be used, particularly if incinerators or solvent recovery are already in place. Solvent free and water free systems also have an important role to play in the future use and applications of acrylic resins for surface coatings.
It should be noted that some of the points already discussed in the proceeding formulation sections, about additives etc., may be mentioned again in the following sections on the principles of specific end use formulations. However, in these instances they will be specific to that particular type of coating system and the reasons why they are relevant for that system.
 6 октября, 2015
6 октября, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 