Before considering waterborne resins in detail, it is necessary to consider the unusual properties of water as a solvent.
Water is the only naturally occurring liquid and also the only one occurring on earth in three physical states, solid (ice), liquid (water) and gas (water vapour).
Water has a molecular weight of 18, but it is a liquid with a relatively high boiling point, whilst most other low molecular weight compounds of hydrogen are gases (e. g. methane, ammonia).
Physical forces of molecular attraction, Van der Waals forces and hydrogen bonding etc, are very large in water and account for its existence in the liquid state at ambient temperatures. The vapour pressure of a liquid is determined by (a) the escaping tendency of a molecules which depends upon the (van der Waals) forces and (b) the number of molecules available. The strong cohesive forces in the bulk give water its high surface tension and low vapour pressure.
Hydrogen bonding between adjacent water molecules can give (H20)n where nisi,2,3 or higher. The basic associated molecules are the dimer, which may be represented as shown in Figure 7-1.
H
H—O—H—O—H
 |
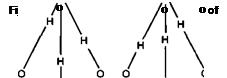 |
Figure 7-1: Dimer of Water
Water has a unique specific gravity/temperature relationship, from 100°C to 4°C the specific gravity increases reaching a maximum at 4°C. From 4°C to -10°C it decreases in specific gravity. Water expands considerably on crystallisation (freezing), 1 kg of ice at 0°C occupies 1090.7 cm. This expansion, of over 9% is unusual since most substances contract on changing from liquid to solid.
Water itself undergoes slight self ionisation and is often represented as shown in Figure 7-3
Figure 7-3: Common Representation of the Ionisation of Water
In reality the ionisation is bimolecular giving a hydroxonium H30+ ion and hydroxyl OH ion, as shown in Figure 7-4.
|
H |
+ |
“ |
|
|
0 + 0 ———————— ► |
6 |
0 1 |
|
|
/ / |
/ |
H |
|
|
H H H H |
H H |
H20 + H20 ^4 H30* + OH
Figure 7-4: Formation of Hydroxonium Ions in Water
Only a small amount of water molecules react as above, depending upon the presence or absence of other ionic species, and equal quantities of H30+ and OH ions are formed, hence, it is a neutral solution.
The differences between water and other solvents are indicated in Table 7-2.
|
TABLE 7-2: SOME PROPERTIES OF WATER AND OTHER SOLVENTS
N/A — Not applicable |
Other differences between water and many common solvents used in resin technology also account for the slow rate of acceptance of waterborne materials in certain application areas and these are outlined below.
(i) Surface Tension
Water was a very high surface tension, considerably higher than the organic solvents used in solvent borne systems as shown in Table 7-3. This high surface tension explains why water has difficulty in wetting metal surfaces.
|
TABLE 7-3: SURFACE TENSION AGAINST AIR AT 20°C VALUES
|
The ease of wetting of a solid substrate may be determined by means of critical surface tension, this is defined as the highest surface tension liquid which will wet the surface. The critical tensions of some common substrates in dynes/cm are given in Table 7-4.
|
TABLE 7-4: CRITICAL SURFACE TENSION OF SOME SUBSTRATES
|
From Table 7-4 it can be seen that water will not wet Teflon, tinplate or steel but may wet glass. In order for water to wet the other substrates, the surface tension of the water must be reduced by the inclusion of surface tension modifiers, which are often termed wetting agents.
 15 сентября, 2015
15 сентября, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 