In the following examples, illustrating the preparation of thermosetting acrylic resins, addition polymerisation of the monomers to form a copolymer is followed by methylolation of the amide group on the acrylamide with paraformaldehyde. By conducting the methylolation in the presence of an alcohol, such as butanol, the methylol groups can be etherified and hence are protected from the possibility of further reaction and crosslinking occurring during manufacture.
Paraformaldehyde is normally used as the source of formaldehyde. Formaldehyde pre-dissolved in butanol (or other alcohol) may also be used. This solution (which is commercially available in the UK and Europe) is a source of both formaldehyde, for the methylolation, and alcohol for the following etherification reaction.
The methylolation and etherification reactions are depicted below:
Acrylamide, incorporated into the polymer backbone reacts with formaldehyde to form methylol acrylamide.
/W^CHj— CH /WNAA
|
c=o I |
+ HCHO |
|
I nh2 |
1
NH
CH2OH Figure 1-82
The methylol groups are capable of self-condensation during the manufacturing process leading to premature branching and cross linking (see section of the book dealing with the chemistry of acrylic monomers).
‘VWSACHj— CH
c=o
NH
I
CHjOH + HO — CH2
I
NH
I
/VWSA QH —- CHjAVWS
AA/vV40H2— CH AVvW
c=o
I
NH
I
CH2— О— CH2
I
NH
I
/WVNA CH — CHj/VWSA
Figure 1-83
In the presence of alcohol the self condensation reaction between the methylol groups is suppressed in favour of an etherification reaction the methylol group and the alcohol.
The etherified methylol groups will not readily undergo further condensation under the conditions pertaining during the manufacturing process.
A/WACH2— CH ‘/V^vA
NH
CH2OH
+ CH3CH2CH2CH2 — HO
1
ЛЛЛЛДСН,—
NH
CH2— О — CH2CH2CH2CH3
+
h2o
Figure 1-84
a) Preparation of Thermosetting Acrylamide Resin (Method 1)
|
TABLE 1-20: FORMULATION FOR A THERMOSETTING ACRYLAMIDE RESIN (METHOD 1)
|
1. Charge monomers and initiators to the monomer tank and mix thoroughly.
2. Charge butanol, butyl glycol and formaldehyde to the reactor, heat to 100°C with the stirrer on to dissolve the paraformaldehyde.
3. Cool to 5(f C and add the tri-ethylamine and acrylamide.
4. Add the premix from the monomer tank as quickly as possible.
5. Add the Solvesso 100 via the monomer tank.
6. Add the fumaric acid.
7. Heat slowly until exotherm begins at about 85°C.
8. Turn off heat and allow exotherm to take the temperature to 120°C.
9. Reflux at 120°C for one hour and then add 80% of the di-tert butyl peroxide.
10. Reflux for a further three hours and add the remainder of the initiator.
11. Check non-volatile content every hour, when 98% plus conversion is obtained, cool and filter.
b) Preparation of Thermosetting Acrylamide Resin (Method 2)
|
TABLE 1-21: FORMULATION FOR THERMOSETTING ACRYLAMIDE RESIN (METHOD 2)
|
1. Dissolve the acrylamide in the solvents in the monomer tank at ambient temperature.
2. Dissolve the paraformaldehyde and tri-ethylamine in the solvents in the reactor.
3. Add 20% of the pre-mix from the monomer tank.
4. Heat to reflux (approximately 110°C).
5. Add the remainder of the pre-mixed monomers over a two hour period.
6. Add 50% of the cumene hydroperoxide and reflux for a further three hours.
7. Add remainder of the peroxide and reflux until non-volatile content is within the desired range.
8. Cool and discharge through a suitable filter.
|
Reactor Charge |
|
|
n-butanol |
25.00 |
|
Xylol |
25.00 |
|
Acrylamide |
3.75 |
|
Paraformaldehyde |
4.50 |
|
Phosphoric acid |
0.15 |
|
Monomer Tank Charge |
|
|
Methacrylic acid |
1.60 |
|
Butyl acrylate |
8.00 |
|
Styrene |
23.00 |
|
Ethyl acrylate |
8.00 |
|
Di-benzoyl peroxide |
1.00 |
|
Total |
100.00 |
|
TABLE 1-22: FORMULATION FOR A THERMOSETTING ACRYLAMIDE RESIN (METHOD 3) |
1. Charge reactor with butanol, acrylamide and paraformaldehyde.
2. To dissolve — heat to 50°C with stirring.
3. Add xylol and phosphoric acid.
4. Heat charge in the reactor to 85°C and start the addition of the monomer charge, over a one hour period. Allow the temperature to rise to 100°C as the result of the exotherm and then continue heating for two further hours up to a maximum of 115°C.
5. Check non-volatile content. If 98% plus conversion, cool and filter.
6. If the non-volatile content is low add a booster shot of di-tert butyl peroxide (0.1%) and continue heating for a further hour, then recheck the non-volatile content, cool and filter as before.
(v) Two Methods of Preparing Thermosetting Hydroxyl Functional Acrylics
a) Preparation of Thermosetting Hydroxyl Acrylic Resin (Method 1)
|
TABLE 1-23: FORMULATION FOR THERMOSETTING HYDROXYL ACRYLIC RESIN (METHOD 1)
|
1. Charge monomer tank and thoroughly mix at ambient temperature. Charge initiator tank.
2. Charge reactor with solvents and heat to 135°C while stirring.
3. Add the monomer charge from the monomer tank over a period of three hours to the solvents in the reactor at 135° C. At the same time feed in the initiator from the initiator tank at a rate to coincide with the monomer feed rate.
4. Hold at 135°C for a further two hours.
5. Add tert butyl perbenzoate if required and hold at 135°C until non-volatile content approaches 50%.
6. Cool and filter via a suitable filter.
1. Charge reactor with toluene and butyl acetate.
2. Charge the monomer tank with stirrer left on to ensure an homogenous mixture. Charge the initiator tank.
3. Heat the solvents in the reactor to 100°C and start the addition of the monomer mixture and the initiator feed.
4. Add the monomers and the initiator over a three hour period, keeping the temperature at 100°C.
5. Hold at this temperature for a further hour and check non-volatile content, continue checking non-volatile content until a value of 50% is obtained (usually in three hours from the end of monomer addition).
6. Cool and reduce to 40% non-volatile content with toluene/butyl acetate (1:1).
1. Filter via a suitable filter.
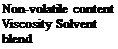 40% ± 2 10 poise at 25°C Toluene/butyl acetate (1:1)
40% ± 2 10 poise at 25°C Toluene/butyl acetate (1:1)
 11 июля, 2015
11 июля, 2015  Malyar
Malyar 
 Опубликовано в рубрике
Опубликовано в рубрике 