Since the overwhelming majority of commercially available water reducible polymers are carboxyl functional, the discussion in this chapter will consider the use of amines. However, the calculations used to determine the quantities of neutralising agent required by a given polymer can be used with modification for acid neutralised systems.
The choice of amine used to neutralise a given polymer system depends on many factors. Amongst those are volatility, ‘solvent strength’, and amine efficiency.
The volatility of the amine must be matched to the mode of use and curing of the final coating. Firstly, it is essential that the amine is volatile, otherwise it becomes entrained within the coating film causing water sensitivity. Secondly, the amine volatility must be matched to the mode of cure, air drying coatings will require a more volatile amine than stoved thermosetting coatings. Finally, the mode of use of the coating must be considered. If a large dipping tank of a water reducible coating were neutralised with a highly volatile amine, it is obvious that the stability of the tank would be extremely limited. The ‘solvent strength’ of the amine is a function of how much the amine is able to behave as an organic solvent contributing towards the effects generated by cosolvents. Figure 7-8 illustrates that for a given amine the viscosity tends to increase with increasing percentage neutralisation.
|
Figure 7-8: Viscosity Variation with Degree of Neutralisation and non-volatile content. |
The ‘solvent strength’ of different amines varies, with dimethyl amino ethanol (DMAE) having more ‘solvent strength’ than triethylamine. Figure 7-9 demonstrates this effect.
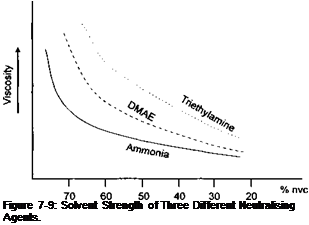 |
As the degree of neutralisation increases, or the solids of the coating reduces, the difference in the ‘solvent strength’ of the amine is of less significance. It is also important to remember that the amine will count towards the VOC of the coating. Therefore, in high water content coatings the ‘solvent strength’ of the amine becomes an important consideration.
The amine efficiency is the amount of amine required to neutralise the polymer system to a specific pH. Amine efficiency may vary from 1 for DMEA to 0.7 for morpholine. The amount of amine required to neutralise the acid functionality of a given polymer may be calculated using the following equation:
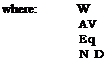 Weight of polymer as base Acid value of base polymer (mg KOH/g) Equivalent weight of neutralising agent Degree of neutralisation і V
Weight of polymer as base Acid value of base polymer (mg KOH/g) Equivalent weight of neutralising agent Degree of neutralisation і V
‘ )
Note that for amine functional systems, the above equation could be used replacing the function AV/1000 x Eq. Wt. KOH by the number of equivalents of amine present per gramme of base polymer.
ГС
а.
=3
ГС
С
С?
Р
Ей
Р
|
|
|
|
 20 сентября, 2015
20 сентября, 2015  Malyar
Malyar 
 Опубликовано в рубрике
Опубликовано в рубрике 