These products are manufactured by copolymerising together in emulsion from two or more monomers, one of which is carboxyl containing. There are a number of options available both in terms of the carboxyl entity and in terms of the comonomers. Components are selected according to ease of copolymerisation and end performance as thickeners.
TABLE 2-2: NON CARBOXYLATED ACRYLIC MONOMERS
Methyl Acrylate
Ethyl Acrylate
N-Butyl Acrylate
2 Ethyl Hexyl Acrylate
Methyl Methacrylate
The usual carboxyl containing monomers are:
• Acrylic Acid
• Methacrylic Acid Methacrylic acid is more hydrophobic.
Polymers may be designed with an essentially linear structure, a branched chain structure or taken to the extreme of developing a 3-dimensional network by crosslinking. This changes the solubility properties when neutralised more towards swollen polymer particles rather than a truly soluble state (Figure 2-8).
|
(a) |
(b) |
(c) |
|
CH — CH2 — CH — CH2 — 1 1 |
0 X 1 -o X 0 1 -o X о X |
CH2-CH2-CH3-CH-CH- 1 1 |
|
1 1 COOH COOH |
1 1 CH2 COOH 1 |
1 1 CH2 COOH |
|
1 CH2 |
1 CH3 1 |
|
|
CH3 |
CH2 CH2-CH2-CH3-CH-CH- |
|
COOH |
Figure 2-8
Variations in Polymer Structure
(a) linear structure, (b) branched structure, (c) crosslinked structure
These polymers are supplied as low viscosity aqueous emulsions in their free acid state (un-ionised). When the pH is increased generally to between 8-10, the carboxyl groups will ionise causing chain expansion and disentanglement which results in significant viscosity increase.
|
Figure 2-9 Viscosity vs pH |
By combining the variants of molecular weight and degree of crosslinking, we can summarise the performance by reference to Tables 2-3 and 2-4.
|
TABLE 2-3: CARBOXYLATED ACRYLIC THICKENERS
|
|
TABLE 2-4: THE EFFECT OF MOLECULAR WEIGHT AND CROSSLINKING ON THE PERFORMANCE OF CARBOXYLATED ACRYLICS
|
In recent years this basic acrylic chemistry has been further extended by the introduction of hydrophobic entities into the polymer backbone resulting in the development of more useful viscosity/rheology properties.
 17 июля, 2015
17 июля, 2015  Malyar
Malyar 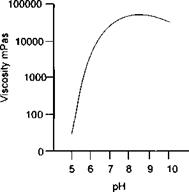
 Опубликовано в рубрике
Опубликовано в рубрике 