Commercially available thermosetting acrylics can be conveniently broken down into 3 main basic chemical types:-
• Acrylamide functional
• Hydroxy functional
• Carboxy functional
1. Acrylamide Based Acrylics
Acrylamide acrylics contain up to 10% of acrylamide (based on solid polymer). The acrylamide is reacted with formaldehyde and the resultant methylol group is etherified with an alcohol such as butanol, as shown in Figure 4-1.
![]()
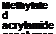 /W*vV С-С-С-С-С-С^ЛЛА
/W*vV С-С-С-С-С-С^ЛЛА
I
nh2
I +
I HCHO
I
I
nhch2oh
I +
I C4H9OH
АЛААЛС-C-C-C-C-CAAW acrytamtde I copolymer
I
NHCH2OC<H9
+ h2o
Figure 4-1
Acrylamide Based Thermosetting Acrylic Resin
This reaction is carried out using a butanol/formaldehyde solution as a single stage in-situ process. Alternatively, it is possible to use an already etherified monomer such as N-isobutoxy methyl acrylamide in the original polymerisation reaction. By this method acrylic polymers with low free formaldehyde levels are produced.
Acrylamide based acrylics are capable of self crosslinking, when subjected to temperatures in excess of 150°C. Crosslinking is a complex mixture of condensation reactions and side reactions involving the liberation of water, formaldehyde, primary alcohol and ethers. The reaction is catalysed by acids. Bisphenol A epoxy resins are often incorporated into acrylamide acrylic coating formulations to improve performance. This epoxy resin also takes part in the curing reaction. Figure 4-2 lists the major reactions which take place.
i) Self Condensation
/VWV4CONH CH2OC, H,
/VWSACONH CH2OC4H9
ДТ^150°- 175°C
АЛЛЛАСОЫН CH2OCH2NHCtyWWN
+ c4h9-o-c4h9
1
<WWS CONH-CH2-NHCOVWSA + HCHO
ii) Self Condensation
AVSMCONH CH2OC4H9
ЛЛИУЛЛСОМН ch2oh
4T^150° — 175°C
ЛЛЛЛЛ conh-ch2-nhco^vw
+ HCHO + C4H9OH
iii) Reaction with epoxy resin
A
^WvV4CH-CH2 + ^VVSACONH CHjOR
ДТ^150°- 175°C
MAMCONH CH2OCH2-CH>VVWN
I
OR
Where R is H or alkyl
Figure 4-2
Crosslinking Reactions of Acrylamide Acrylics.
 9 августа, 2015
9 августа, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 