The “glass transition temperature” (Tg) of a polymer is a 2nd order transition, involving a change in the solid polymer, from a flexible state to a rigid state.
There are a number of transitions, depending upon the complexity of the molecular structure, for example the temperature at which rotation stops for a styrene unit about its axis in a polystyrene polymer is a transition point. However the glass transition point or Tg is the temperature at which all molecular movement including mobility about the bonds in the backbone of the polymer becomes frozen.
Although Tg increases with molecular weight, it is largely a constant, for all practical purposes, over a range of molecular weights normally encountered with acrylic surface coatings. Crosslink density affects the Tg by several degrees per mole percentage of crosslinks. The chemical structure has the greatest effect on Tg, large changes being observed as the chemical structure is modified. In general the longer the side chain of a polymer the lower the Tg, whereas the presence of methyl groups on the carbon atom (as with methacrylates) increases the Tg by increasing the propensity for steric hindrance. Branching of the side chain also raises the Tg. This is illustrated in the table below, which shows Tg values for some common acrylic homopolymers.
|
HOMOPOLYMER |
Tg°K |
|
poly (acrylic acid) |
379 |
|
poly (acrylamide) |
438 |
|
poly (ethyl acrylate) |
249 |
|
poly (n-butyl acrylate) |
219 |
|
poly (2-ethylhexyl acrylate) |
223 |
|
poly (methyl methacrylate) |
378 |
|
poly (styrene) |
373 |
|
poly (n-butyl methacrylate) |
295 |
|
poly (2-hydroxy propyl acrylate) |
266 |
|
poly (ethyl acrylate) |
338 |
|
poly (methyl acrylate) |
282 |
|
poly (2-hydroxy ethyl acrylate) |
258 |
|
poly (2-hydroxy ethyl methacrylate) |
328 |
|
poly (iso-butyl methacrylate) |
321 |
|
poly (2-hydroxy propyl methacrylate) |
346 |
Almost any intrinsic property of a polymer which is temperature dependent can be used to measure the Tg of the polymer. Properties which may be used include specific volume, specific heat and refractive index, etc.
The measurement of Tg involves taking readings of the property, over a range of temperatures, either side of the Tg.
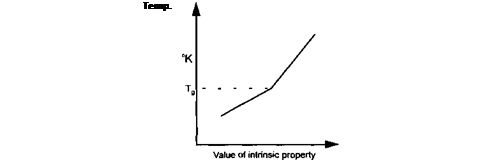 |
A plot of temperature against the property value will produce a graph, the slope of which shows an inflexion at the Tg.
Instrumental methods are also available for determination of Tg with one of the commonest being the use of a differential scanning calorimeter (DSC).
 2 июля, 2015
2 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 