Originally developed commercially in the late 30’s by Castan, and early 40’s by Ciba A. G., then taken up for use in aircraft manufacture, epoxy (polyglycidyl ether) technology was introduced to the coatings industry in the late 1940’s. The excellent chemical and physical properties exhibited by epoxies has meant a sustained and continued growth in importance within the coatings industry to a level such that over 500,000 tonnes/year of epoxy resins were consumed by the industry in 1990.
Each major manufacturer (e. g. Ciba Geigy, Dow, Shell, Du Pont) of epoxy resins has an extensive range of products based on the glycidation of epichlorohydrin (EPC) and polyhydroxy phenols (or polybasic acids). The most significant series of resins for the can coatings industry is based on the condensation reaction of EPC and diphenylolpropane (4,4-isopropylidenediphenol, Bisphenol A) as illustrated in Figure
J n
Polyglycidyl ether of Bisphenol A
Figure 2-18
Polyglycidyl Ether of Bisphenol ‘A’
The variations produced by the above reaction are almost unlimited. Practically, however, the range of resins offered by the manufacturers are restricted to those most commercially viable. Although each manufacturer will have their own commercial names or codes for their products, in order to differentiate between the variants possible, most coatings technologies generally refer to the ‘Epikote’* series of epoxy resins when discussing resin types. A brief example of the resins and their epoxy functionality are listed in Table 2-6.
|
TABLE 2-6: EPOXY RESINS WHICH CAN BE USED IN INTERNAL LACQUERS
* Epikote is a trade mark of Shell Chemicals |
Note that there are significant variations in the EEW (epoxy equivalent weight) or EGC (epoxy group content) between the same grades of epoxy resin (for example the EEW for a ‘g’ type epoxy may range from 260 — 440).
Using the above codes, the technologists can discuss their general requirements without having to refer to specific technical data. As previously stated, the range of epoxies available off the shelf, although suitable for most applications, can be restrictive to the coatings technologist. However, a process has been developed whereby chemists can produce epoxy resins tailored to their individual requirements. The process uses a low molecular weight liquid epoxy reacted with DPP with a catalyst under dry nitrogen to produce a chain extended high molecular weight solid epoxy. This is often referred to as advancing an epoxy resin or epoxy advancement. The reaction is as shown in Figure 2-19.
Higher Molecular Weight Bisphenol ‘A[5] Epoxy Resins
Figure 2-19
The Preparation of Higher Molecular Weight Bisphenol ‘A’ Epoxy Resins The molecular weight and EEW can be controlled and optimised by varying the ratio of liquid resin to DPP. The molecular weight (MW), and epoxy equivalent weight (EEW, or WPE, weight per epoxy) can be calculated and manufactured to individual requirements using the following calculations:
100 _ X _ Y
EEWi “ EEW2 HEW
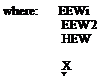
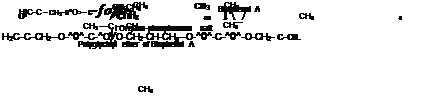 |
desired epoxy equivalent weight of extended epoxy, epoxy equivalent weight of liquid epoxy to be extended, hydroxyl equivalent weight of Bisphenol A, (or alternative polyhydroxyl phenol)
% diglycidyl ether of Bisphenol A % Bisphenol A
The coatings have a number of criteria to meet in order to satisfy the can manufacturers’ requirements, particularly in the two piece beer can market, as the flavour of beer is easily impaired. A 5-10 ppm iron contamination of a beer packed in a two piece electro tin plated steel can will impart a metallic flavour to the product. It is therefore very important that the internal coating provides a protective dry film of high integrity. The following criteria are general requirements which must be met by the coating formulator:
 25 июля, 2015
25 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 