The degree of polymerisation, i, is defined as the number of monomer units in a polymer molecule.
Assuming that little or no transfer occurs then the relationship between v and і will depend on the mode of termination.
a) Termination by combination — two radicals of kinetic chain length v combine to form one molecule with a degree of polymerisation i, that is:
і = 2v
b) Termination by disproportionation — two radicals of kinetic chain length v react to form two molecules each with a degree of polymerisation i, that is:
і = v
Since, as we have seen above
= к2 [M]2 2kt Rp
and for the steady state
and
Rp = kp[M](^),/2
it follows that the rate constant for propagation kp (in the early stages of the reaction) will be proportional to [I] 1/2
If f (the initiator frequency or efficiency) is independent of [M] then the rate of propagation is proportional to [M]
that is:
RP a [M]
This holds experimentally where the initiator frequency f is high.
Where f is low then:
f a [M]
and
RP a [Mf2
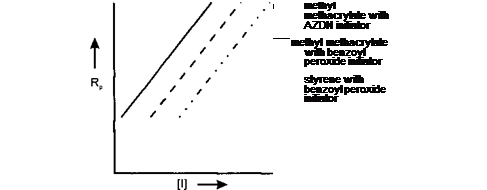 |
Plots of Rp versus [I] give a straight line graph with a slope approximately 0.5 as illustrated below:
If the initiator concentration does not vary much during polymerisation (as for example when using a continuous addition or ‘drip feed’ process) and the initiator frequency f is independent of the monomer concentration (as is the case when f approaches 1) then
Rp a [M]
and the polymerisation will proceed in accordance with the principles of first order kinetics.
An example is included here to illustrate this.
Methyl acrylate is polymerised in xylene at 50°C using azo di-isobutyronitrile (AZDN)
If [I] = 1 x 10’3 mole litre"1
Initiation rate constant kd = 1.2 x 10 sec"1
7 -1 -1
Termination rate constant kt = 7.2 x 10 litres mole sec Calculate [M ] when f=l
m
. _ [1 x1.2×10“5×1 хЮ-3]72
[M’j = ———————— Tv————
[7.2 x 10′]’
= 1.28 x 10-8 molelitres-1
Under the conditions of polymerisation [M ] reaches a maximum value within a few seconds of the start of the reaction and thereafter remains constant as illustrated below.
|
Figure 1-20 |
The following table taken from the literature compares [M] and [I] for two monomers commonly encountered in acrylic polymers. The initiator is AZDN and a reaction temperature of 50°C was quoted.
|
TABLE 1-3
|
 24 июня, 2015
24 июня, 2015  Malyar
Malyar 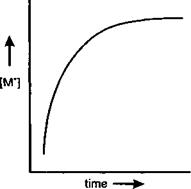
 Опубликовано в рубрике
Опубликовано в рубрике 