The solvents used in water reducible coatings are usually polar and are fully or partially water miscible. These are alcohols and glycol ethers. Those based on ethylene glycol have been the most commonly used, but are now being superseded by propylene glycol based glycol ethers because of their reduced toxicity. Different levels and types of cosolvent have considerable effect on the viscosity of the coating system as shown in Figure 7-10.
|
Figure 7-10: Effect of Cosolvents on Viscosity |
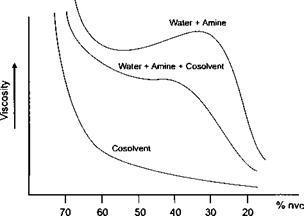
The actual relationships between the amounts of cosolvent and water in the system and the degree of neutralisation and the ‘solvent strength’ of the amine are very complex and are in turn dependent on the molecular weight of the polymer. In general, as the polymer molecular weight is increased, the film properties of the coating are improved but solubility is reduced, often requiring an increased acid value to obtain solubility. Overton et al(1) illustrated some of the factors affecting this relationship, as shown in Figure 7-11.
The whole phenomenon becomes more complex when one considers that the viscosity solids relationship is effected by the temperature at which the polymer is neutralised and thinned. The optimum temperature for neutralisation is approximately 70°C.
Water is slow to evaporate compared to many organic solvents®, and this phenomenon could cause problems if water were to remain in the film as the ’last solvent’. For this reason it is common practice to use a proportion of lower boiling solvent which can remove water from the film as an azeotropic mixture with itself. It is also common to find a small percentage of hydrocarbon in the solvent mixture. This acts to reduce the surface tension (often very high in these polar mixtures) at the air/liquid interface, and it may also act as an antifoaming agent.
|
Figure 7-11: Illustration of the Relationship of Water, Water Amine, and Water Amine and Cosolvent, on Viscosity*1* |
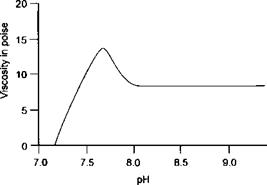
The dependence of viscosity upon pH in the presence of cosolvent and water can be demonstrated by considering example 7 in Chapter II (IV) — the water soluble acrylic copolymer prepared as an emulsion and solubilised by ammonia into an aqueous solution. Such solutions are termed ‘colloidal’ in most supplier’s literature. The molecular weight of a polymer of this type is typically between 5000 and 30,000 which is rather low for a colloidal particle. It is probable that several molecules associate to form structures similar to the micelles formed by surfactants. A micelle of sodium stearate is believed to comprise 50-100 molecules of the soap. The same number of ionic groups would be given by 4-8 molecules of a copolymer of molecular weight 10,000 containing 10% by weight of acrylic acid. The viscosity of the polymer varies with the pH as shown in Figure 7-12.
|
Figure 7-12: Viscosity of a Water Soluble Acrylic Polymer as a Function of pH; 30% Solids in 3:1 Water:Isopropanol |
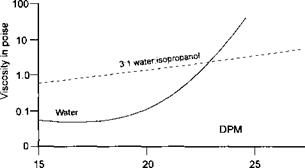
This particular polymer is insoluble below a pH of 7 and remains in emulsion with a low viscosity. A maximum viscosity occurs at pH 7.5 which indicates that the individual molecules have an extended form caused by the repulsion of the anions. On adding further ammonia, the ‘micelle’ structure is formed and the repulsion of the anions is reduced by the presence of ammonium gegenions. At lower solids, the viscosity maximum may occur at slightly higher pH (7.6-7.7). The effect of solvent composition on viscosity is shown in Figure 7-13. The explanation of the reason why a relatively small amount of isopropanol should have such a marked effect on the shape of the curve is not easy to understand, but it depends upon the alcohol affecting the double layer of electrical charge.
|
Non-volatile content, % |
Figure 7-13: Variation of Viscosity with Concentration for an Alkali Soluble Acrylic
Copolymer
The very sharp increase in viscosity above 22% solids can cause problems during application if some water evaporates. The addition of alcohol, normally 5-30% by weight of the water used, will maintain the viscosity over a wider solids range and it will also permit higher solids coatings to be applied compared to a system only containing water. Waterborne stoving finishes use the higher boiling amines, often tertiary amines, such as dimethyl aminoethanol, unlike some ambient finishes which use ammonia. With ammonia, amides are formed. Also ammonia is volatile and as it evaporates the pH of the coating varies, affecting viscosity and film weight. An additional problem is that many people do not like ammonia odours. The use of a low volatility tertiary amine gives a more stable coating in the roller coater. Tertiary amines avoid amide formation which occurs with ammonia. An alternative to ammonia for many ambient systems is triethylamine which obviates amide formation.
 20 сентября, 2015
20 сентября, 2015  Malyar
Malyar 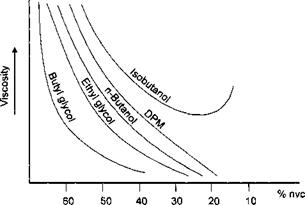
 Опубликовано в рубрике
Опубликовано в рубрике 