|
-r CH2— CHX-j — CH2— c L -*n-1 |
The radical formed by abstraction above is available to initiate propagation of another polymer chain.
|
CH2— |
CHX- |
L CH2- |
CHX |
+ |
RSH |
|
mercaptan |
|||||
|
1 |
r |
||||
|
CH2— |
CHX- |
b CH2- |
X о |
+ |
RS |
|
*n |
mercapto radical |
The effectiveness of a chain transfer agent is measured in terms of its ability first to terminate a growing polymer chain, and then to initiate the propagation of another polymer chain.
This is quantified as the transfer rate constant Cs, which is temperature dependent and is different for different monomer types.
In general, long chain alkyl mercaptans give the best performance as CTA’s. They are extensively used in commercial polymerisations of acrylate monomers as modifiers to control the molecular weight of the final polymer.
The number of chain ends arising from transfer reactions can be obtained from the equation:
![]() Ns = 2Cs
Ns = 2Cs
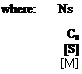 number of chain ends containing modifier per monomer unit in the polymer.
number of chain ends containing modifier per monomer unit in the polymer.
rate co-efficient of transfer for the modifier molecular concentration of modifier molecular concentration of monomer
This has been related empirically to the molecular weight of the polymer by Mayo in his equation:
1 = Cs [S] _1I_
P [M] P0
where: P = degree of polymerisation in presence of modifier
Po = degree of polymerisation without modifier
Cs, [S] & [M] are as before
|
TABLE 1-2: COMPARISON OF Cs FOR VARIOUS COMMONLY ENCOUNTERED CTA’s
|
 21 июня, 2015
21 июня, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 