A wide range of rheology modifier additives is commercially available for solventborne coatings. Some important classes are discussed in the following chapters.
Organoclays are the most widely used inorganic thickeners in the paint and coating industry. They are derived by modification of naturally occurring laminar silicates (clays) such as hectorite and bentonite. They have replaceable cations (Na+, Ca+ or Li+) on the surface of the crystal that are replaced, through an ion exchange reaction, by organic quaternary ammonium cations such as [dimethyldioctadecylammo — nium]+ or [dimethylbenzyloctadecylammonium]+. Occasionally a blend of quaternary amines is used to tailor the properties of the product.
Organoclays are supplied as a fine powder having agglomerated stacks of platelets. They must be added to the paint during the dispersing stage. Before addition, adequate wetting, deagglomeration
|
|
and activation should be accomplished in an appropriate solvent. Under the strong shear force and in the presence of a polar solvent such as alcohol, propylene carbonate or water as an activator, the stacks break up into individual platelets with simultaneous solvation of the organic cations on the planar surfaces. The threedimensional gel structure developed via hydrogen bonding through the hydroxyl groups at the edges of the organoclay platelets results in thickening of the paint (Figure 5.7). The structure is reversible; it breaks down easily under low-shear forces and can be regenerated when the shear force is removed. This process explains the mechanism for development of thixotropic consistency by organoclays.
Rapid recovery of viscosity, which is useful for sag control, good pigment suspension at low dosage level, and temperature insensitivity are the key advantages of organoclays. Some of the limitations associated with organoclays are reduced gloss and flow at excessive dosage and possible reduction in corrosion resistance due to presence of bromides or chloride ions as impurities.
 23 декабря, 2015
23 декабря, 2015  Pokraskin
Pokraskin 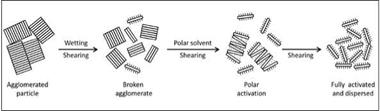
 Опубликовано в рубрике
Опубликовано в рубрике 