UV absorbers do not protect the surface of coatings, and therefore, they are often supplemented by another type of light stabilizers called radical scavengers. In contrast to UV absorbers, radical scavengers act at the coating’s surface as well as in bulk, and act only when free radicals are generated. They are based on ste — rically hindered amines, most commonly known as hindered amine light stabilizers (HALS). All the technically important hindered amine light stabilizers have the 2,2,6,6-tetramethylpipe — ridine group as their basic structure.
Even the small amount of UV light that is not absorbed by a UV absorber will generate free radicals capable of starting the degradation process. Hindered amine light stabilizers scavenge these radicals to protect the polymer from degradation. In the process, under the influence of light and oxygen, these light stabilizers form a nitroxy radical as an active agent, which reacts with polymer radicals to produce an amino ether. The generated aminoether in turn consumes the peroxy radical to regenerate nitroxy radical. As all the active radicals, including peroxy radicals, are transformed to non-free radical compounds, the degradation reaction is terminated. A simplified scheme showing the mode of action of hindered amine light stabilizers is shown in Figure 5.21.
|
|
|
|
Typically hindered amine light stabilizers are used up to 3 % by mass of coating, depending on other formulation parameters such as type of binder and pigments. A combination of UV absorbers and hindered amine light stabilizers are often used in demanding applications due to their synergistic effect.
 21 января, 2016
21 января, 2016  Pokraskin
Pokraskin 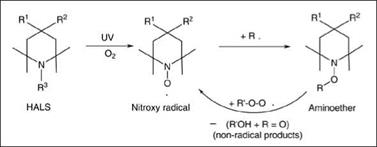

 Опубликовано в рубрике
Опубликовано в рубрике 