After azo pigments, the second most important class of pigments in terms of usage is copper phthalocyanine and its derivatives because of their excellent overall performance properties combined with good economy. They are ideal pigments, having high color strength, excellent thermal stability, good light fastness and excellent solvent fastness. They cover the blue and green range of the color spectrum.
Phthalocyanine blue pigments are polymorphic, existing in at least five crystal forms, but commercially important crystal forms include the reddish-blue а-form, the greenish-blue p — form, and the occasionally used intense reddish-blue є-modification. Phthalocyanine green pigments with a bluish to yellowish undertone are produced by introduction of chlorine or bromine atoms into the phthalocya — nine molecule. Crude phthalocyanine blue pigment is normally in the p — form, with coarse particles. To produce commercially useful fine particles of different crystal modifications, different finishing technologies are used. The unstabilized а-form reverts to the p-modification under action of heat or aromatic solvents; therefore, it is modified by partial chlorination or certain additives to form stabilized grades. Due to very small particle size, phthalocyanine pigments have a tendency to flocculate, so flocculation-stable grades are prepared by special surface treatment. Different types of phthalocyanine pigments are Pigment Blue 15 (unstabilized а), Pigment
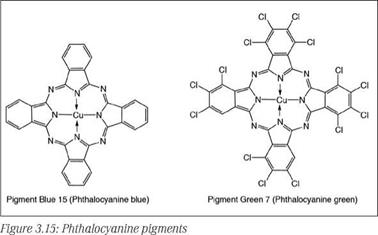
Blue 15:1 (stabilized a), Pigment Blue 15:2 (non-flocculating stabilized a), Pigment Blue 15:3 (P), Pigment Blue 15:4 (non-flocculating P), Pigment Blue 15:6 (є) and Pigment Green 7 and 36. Examples are shown in Figure 3.15.
 28 ноября, 2015
28 ноября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 