UV curing is an environmentally friendly coating technology in which binder is cured on exposure to UV radiation via either free — radical copolymerization or cationic polymerization. The vehicles of UV-cured coatings contain oligomeric resins and reactive diluents. The main resin types for such coatings are radically polymerizable unsaturated polyesters and acrylates or methacrylate terminated oligomers of polyepoxides, polyesters, polyurethanes and polyethers, as well as epoxies and vinyl ethers that are cured cationically. The UV-curing process invariably requires generation of free radicals or cations that initiate polymerization and
|
|
curing reactions. Photoinitiators, also known as UV initiators,
are compounds capable of absorbing UV radiation and generating active species such as free radicals or cations. Therefore, they are the essential additives in any UV-curable coating composition. Photoinitiators are classified into two major classes; photoinitiators for radical polymerization and photoinitiators for ionic polymerization.
Free-radical photoinitiators are further divided into two types:
• Those that undergo intramolecular bond cleavage, known as homolytic fragmentation type or Type I photoinitiators (a-cleavage type). Examples are benzoin ethers, substituted acetophenone derivatives, acyloxime esters, benzil ketals, and cyclic benzoin and benzils. Representative examples are shown in Figure 5.15.
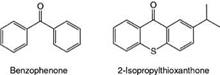
• Those that undergo intermolecular H-abstraction from an H-donor, known as H-abstraction type or Type II photoinitiators (non-fragmentation type). Tertiary amines with abstractable a-H atoms have been shown to be particularly effective as synergists (H-donors). Type II photoinitiators include benzophenone, Michler’s ketone,
|
|
thioxanthones, benzils and quinines. Representative examples are shown in Figure 5.16.
 16 января, 2016
16 января, 2016  Pokraskin
Pokraskin 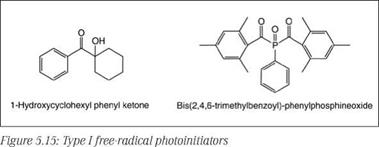
 Опубликовано в рубрике
Опубликовано в рубрике 