Novolacs are phenolic resins prepared under acid catalyzed conditions with a molar excess of phenol (f:p <1), see Figure 2.21. Acids such as oxalic acid, sulfuric acid, hydrochloric acid, formic acid, and aromatic sulfonic acids are used as catalysts. In contrast to resoles, here the methylolated phenol molecules are rapidly polymerized through methylene linkages rather than ethers. To control the reaction in larger scale production, normally formaldehyde is gradually added over time.
|
Figure 2.21: Chemistry of novolac resins |
As novolac resins do not have any reactive methylol groups, they are thermoplastic in nature, but with further addition of aldehyde or resole, they are converted to a cross-linked insoluble polymer. Novo — lacs can be considered reactive resins with respect to their phenolic groups, which can be used to cure epoxy resins or be converted to glycidyl ethers to produce epoxy resins (Section 2.9.1). Novolacs prepared from unsubstituted phenol and formaldehyde are hard, brittle, amber colored products that are soluble in polar solvents and are not of much significance in the coating industry. However, more linear resins are formed by using substituted phenols, generally alkyl phenols, with reduced functionality of the phenolic monomer, which leads to a less branched structure. Novolacs useful in the coating industry are of the following types:
Alcohol-soluble non-heat-reactive types are low MW novolacs derived from phenol or cresols that are used in the preparation of novolac epoxy resins, epoxy curing agents, epoxy-phenolic systems, and powder coatings.
Oil-soluble non-heat-reactive novolac phenolic resins are produced from a substituted phenol such as p-phenylphenol, p-tert-butyl — phenol or p-nonylphenol with a lower f:p ratio. They are designed to be used with drying oil-based varnishes as hard components. Due to the availability of a larger variety of synthetic varnishes, the usage of such varnishes has been declining fast.
 19 сентября, 2015
19 сентября, 2015  Pokraskin
Pokraskin 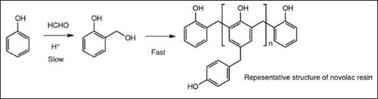
 Опубликовано в рубрике
Опубликовано в рубрике 