Despite the wide range of properties of unmodified alkyd resins mentioned above, alkyds suffer from some inherent limitations. In general, coatings based on alkyd resins have poor water, alkali and chemical resistance, yellowing tendency and poor exterior durability and gloss retention properties. In order to partly overcome these limitations, alkyds are frequently chemically modified. Chemical reactions with alkyd resins can take place via their hydroxyl groups, carboxyl groups or the double bonds of their unsaturated fatty acids. Though it is out of the scope of this book to discuss each of the modifications in detail, some of the most common ones are listed in Table 2.3 with their characteristic advantages.
|
Table 2.3: Common modified alkyd resins
|
It should be noted that while these modifications will improve certain properties, they may also cause a compromise with other properties. For example, rosin modification causes brittleness, poor color retention and water sensitivity of the film. In vinylated alkyds, due to reduced residual unsaturation after grafting, the cross-link density is lower compared to the unmodified alkyd, and hence solvent resistance is reduced. Such resins also have an increased tendency for yellowing. Certain modifications, such as by urethane, silicone or acrylic components, increases the cost of the resin, rendering them only suitable for specific applications that justify their higher cost.
The modifications with urethane and silicone are discussed in Section 2.11.3.2 and 2.12.5 respectively. Alkyd resins are modified by reacting them with polyamide resins to achieve special rheological properties (Section 5.3.2.5) and are called thixotropic alkyds. Although epoxy esters are a class of epoxy resin, they are frequently considered alkyds rather than epoxies. They are also ester-based resins, but are derived by esterification of a secondary hydroxyl and oxirane group of epoxy resin and vegetable oil fatty acids. This class of resins is discussed in Section 2.9.2.1.
Vinylated alkyds are produced by copolymerization of unsaturated monomers such as styrene, vinyl toluene, a-methyl styrene and methyl methacrylate with an alkyd resin in the presence of free radical initiators such as benzoyl peroxide or di-tert-butyl peroxide. This modification involves free radical-initiated addition polymerization of the vinyl monomers in the presence of the alkyd resin, resulting in grafting of vinyl chains onto the fatty chains of the alkyd (at the unsaturation sites of alkyds). Grafting is favored on fatty chains with conjugated double bonds compared to unconjugated fatty chains. Type of initiator and temperature are also important factors for grafting efficiency. The mechanism of grafting is depicted in Figure 2.12.
|
|
 5 сентября, 2015
5 сентября, 2015  Pokraskin
Pokraskin 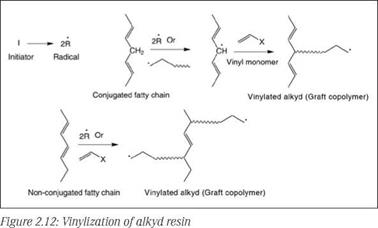
 Опубликовано в рубрике
Опубликовано в рубрике 