Safe preparation of polyester resins from polyfunctional monomers (Fn > 2) without gelation requires careful optimization of average functionality and calculation of the degree of polymerization at the gel point (Pgel). Wallace Carother and P. J. Flory have made significant contributions in this area; however, a detailed discussion is outside the scope of this book.
A wide range of raw material is available to design polyesters with a broad spectrum of properties. In addition to the polyols listed in the section on alkyd resins (Section 2.3), other specialty diols are also used in polyester synthesis. Some important polyols are listed in Table 2.4.
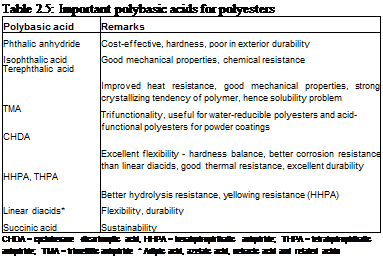
Various polybasic acids are used in polyester synthesis, including aromatic acids as well as aliphatic acids. Some important acids used in polyester synthesis and their primary characteristics are shown in Table 2.5.
In addition to polybasic acids, some monobasic acids including lower MW synthetic branched fatty acids and monobasic aromatic acids are also used for controlling functionality and MW of polyesters.
|
Table 2.4: Important polyols for polyesters
Fn = number average functionality; TMPD = 2,2,4-trimethyi-1,3-pentanedioi; CHDM = cyclohexane dimethanoi; BEPD = 2-butyi-2-ethyi-1,3-propanedioi; HBPA = hydrogenated bisphenoi A; TMP = trimethyioi propane; TME = trimethyioi ethane |
 |
Some examples of such monobasic acids are benzoic acid, p-tert — butylbenzoic acid, hexahydrobenzoic acid, 2-ethylhexanoic acid and isononanoic acid.
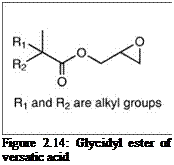 Another interesting material often used in polyester resins is the glycidyl ester of versatic acid (Figure 2.14), a synthetic saturated monocarboxylic acid of highly branched C10 isomers. The highly branched alkyl group provides excellent wetting, exterior durability, hydrophobicity, flexibility and impact resistance, and acid etch resistance, and low viscosity.
Another interesting material often used in polyester resins is the glycidyl ester of versatic acid (Figure 2.14), a synthetic saturated monocarboxylic acid of highly branched C10 isomers. The highly branched alkyl group provides excellent wetting, exterior durability, hydrophobicity, flexibility and impact resistance, and acid etch resistance, and low viscosity.
 8 сентября, 2015
8 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 