Being a glycidyl ether of a novolac type phenol formaldehyde resin, epoxy phenol novolac resins (Figure 2.39 (a)) are the polymeric multifunctional counterpart of bisphenol F resin. They are supplied as highly viscous through semisolid to solid resins. They are generally prepared by reacting epichlorohydrin with novolac type phenol formaldehyde resins.
|
Figure 2.39: Some multifunctional epoxy resins: (a) epoxy phenol novolac, (b) triglycidyl isocyanurate, (c) aminophenol epoxy resin, (d) tetraphenole- thane epoxy |
The maj ority of commercial novolac epoxy resins are available with average functionality ranging from around 2.2 to 4, though higher functionality (up to ~6) is used when required. Unlike BPA epoxy resins, these resins do not have hydroxyl functionality. Due to higher functionality, they can produce films with a highly cross-linked matrix having high heat and solvent resistance and resistant to aggressive chemicals. With proper selection of cross-linking agent, these networks can even resist mineral acids such as H2SO4, HCl or HF.
Some examples of multifunctional epoxy resins other than epoxy phenol novolac resins are depicted in Figure 2.39 (b), (c) and (d). Triglycidylisocyanurate is a solid trifunctional epoxy cross-linker used in powder coatings, which provides a higher cross-link density and superior photochemical stability compared to BPA epoxy resins. The use of triglycidyl isocyanurate may present toxicity hazards.
 1 октября, 2015
1 октября, 2015  Pokraskin
Pokraskin 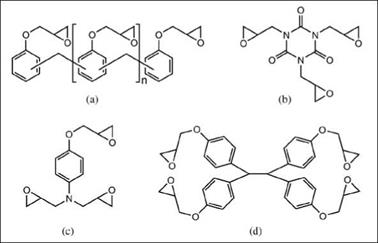
 Опубликовано в рубрике
Опубликовано в рубрике 