In the absence of repulsive forces, freshly disrupted pigment particles would recombine (flocculate) in response to continuous collisions between them due to Brownian movement and attractive forces, such as London van der Waals forces, between them. The flocculation of pigment particles leads to gloss reduction, loss of opacity, poor color development, inconsistent color, and defects such as settling, syne — resis, flooding and floating, leading to poor paint performance. The process of dispersion stabilizes the particles by establishing enough repulsive forces between the separated particles to prevent them from flocculating. The stabilization is induced by using dispersing agents, which operate by a mechanism of charge stabilization or steric stabilization.
In charge stabilization (electrostatic stabilization), an anionic or cationic dispersing agent is adsorbed onto the pigment surface and forms an electrical double layer around the
pigment particles (Figure 5.1). The tendency to flocculate is reduced by electrostatic repulsion between pigment particles, as they all carry the same charge, and the dispersion is stabilized. Charge stabilization is an especially useful mechanism in waterbased systems. The dispersing agent must be ionic in nature to establish this mechanism.
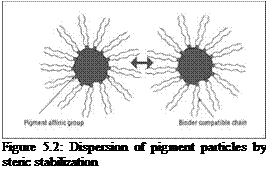 In steric stabilization (entropic stabilization), the repulsion between particles is achieved by steric hindrance arising from the adsorbed layer of dispersing agent (Figure 5.2). The pigment affinic group, also known as the anchoring segment, of the dispersing agent is selectively adsorbed on the pigment surface, while the rest of the segment of the dispersing agent, consisting of long resin — compatible chains, are solvated and protrude into the binder phase. As pigment particles approach each other, these adsorbed polymeric chains intermingle, and in so doing, they lose a degree of freedom, causing a reduction in entropy, which is unfavorable and provides the necessary hindrance to prevent further attraction. Steric stabilization is an important mechanism applicable in both solventbased and aqueous systems. The effectiveness of this type of stabilization is dependent on the thickness of the adsorbed layer, the structure of the adsorbed layer and whether there is enough coverage of the pigment surface by the dispersing agent (adsorption density).
In steric stabilization (entropic stabilization), the repulsion between particles is achieved by steric hindrance arising from the adsorbed layer of dispersing agent (Figure 5.2). The pigment affinic group, also known as the anchoring segment, of the dispersing agent is selectively adsorbed on the pigment surface, while the rest of the segment of the dispersing agent, consisting of long resin — compatible chains, are solvated and protrude into the binder phase. As pigment particles approach each other, these adsorbed polymeric chains intermingle, and in so doing, they lose a degree of freedom, causing a reduction in entropy, which is unfavorable and provides the necessary hindrance to prevent further attraction. Steric stabilization is an important mechanism applicable in both solventbased and aqueous systems. The effectiveness of this type of stabilization is dependent on the thickness of the adsorbed layer, the structure of the adsorbed layer and whether there is enough coverage of the pigment surface by the dispersing agent (adsorption density).
 20 декабря, 2015
20 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 