Chemically, all alkyds are polyesters and are derived from a polycondensation reaction of polyhydric alcohols and polybasic acids with a certain degree of modification by some kind of oil or fatty acid. Among the important chemical reactions involved in alkyd resin technology are direct esterification of carboxylic acids with alcoholic hydroxyl groups, transesterification reactions, and reaction of an alcoholic hydroxyl with an acid anhydride. These reactions are shown in Figure 2.7.
Transesterification can occur either by alcoholysis or by acidolysis; the latter is less important commercially. When acid anhydrides are used, they react in two steps: first, the anhydride ring opening by a hydroxyl, giving a half ester of acid, followed by condensation of the acid group with another hydroxyl. The first reaction occurs at
|
|
relatively low temperature, as it does not generate any water as a byproduct that needs to be removed in order to get a high yield of resin; therefore, the most important commercial route is to use anhydrides whenever possible. In addition to the desired esterification reaction, a competing side reaction is etherification by condensation of two alcohol groups.
Alkyds are made by different processes, depending upon the available raw materials, final product properties and cost. The alcoholysis process (also called the monoglyceride process) or acidolysis process is used when oil is one of the starting materials, while direct esterification uses fatty acids as starting materials, with better control of final resin properties, but generally at higher cost.
 28 августа, 2015
28 августа, 2015  Pokraskin
Pokraskin 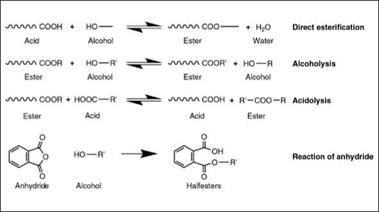
 Опубликовано в рубрике
Опубликовано в рубрике 