1.2.1 Introduction to polymers
In the previous chapter, binders or film formers were described as one of the most important components of paints and coatings. Binders are essentially polymeric materials and thus presenting some concepts and definitions related to polymers will be useful in understanding the different types of binders used in paints and coatings.
 |
Simply put, polymers are giant molecules. Unlike small molecules such as H2O, benzoic acid, and glucose that we are more familiar with, polymers are large molecules with a chain-like structure. This long chain-like polymer structure is formed by bonding between small molecular units called monomers. The chemical process by which monomers bond with each other and form long chain-like structures is a reaction called polymerization (Figure 2.1).
Figure 2.1: Simplified schematic representation of polymerization reaction
It should be noted that in a polymerization reaction, many polymer chains are formed, and these chains are not all of the same size, but rather a distribution of chains having a different chain length.
V. Mannari, C. J. Patel: Understanding Raw Materials © Copyright 2015 by Vincentz Network, Hanover, Germany ISBN: 978-3-86630-603-5
The average number of monomeric units in a sample of a polymer is known as the average degree of polymerization, expressed as DP bar. Similarly, the molecular weight (MW) of a polymer sample is also calculated by averaging the MW of individual chains, which is expressed as the average MW, or Mn bar. A number average MW can be calculated by multiplying the number average DP bar value with the MW of the repeat unit structure.
Equation 2.1: Mn = DP * MW of repeat unit structure
Depending on the type of monomers and the polymerization process, the average MW of polymers can be from a few thousands to hundreds of thousands of grams per mole. Polymers with very low average MW, on the order of a few hundreds grams per mole, are frequently referred to as oligomers. Many polymers are available in nature or synthesized by living organisms and are called natural polymers, whereas those prepared from monomers by polymerization reactions are called man-made or synthetic polymers. Both natural and synthetic polymers are used in paints and coatings. In the paint and coating industry, the term resin is frequently used for polymeric materials. In this book, the terms polymers, resins, and binders are used interchangeably.
There are two basic types of polymerization reactions, as briefly described below.
Chain-growth polymerization (addition polymerization)
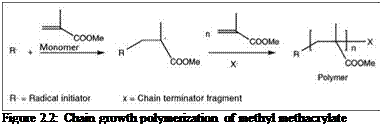
In this process, high MW polymers are formed by chemical bonding between monomers through a very fast polymerization reaction. This polymerization process requires an initiator compound that produces active species, such as free radicals or ions, which initiate the polymerization reaction. Once initiated, monomers combine to form a growing chain with a large number of monomer units, called a propagation step. Propagating chains then terminate by a number of different routes. Figure 2.2 shows polymerization of methyl methacrylate monomer to poly(methyl methacrylate) by chain-growth polymerization. The most common polymers used in paints and coatings that are prepared by this process are acrylics and vinyl polymers.
 |
Step-growth polymerization (condensation polymerization)
In this type of polymerization, monomers with at least two functional groups are used, and the linkages between monomeric units are formed by reaction between the functional groups of monomers that generally produce a low MW by-product, such as water. The polymer MW grows in a step-wise manner at a much slower rate compared to that for chain-growth polymerization. This type of polymerization does not produce a very high MW polymer. Figure 2.3 shows a polyesterification reaction, an example of step-growth polymerization.
Figure 2.3: Example of step-growth polymerization
 21 августа, 2015
21 августа, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 